1. Background
Mammography (MMG) and breast ultrasonography (US) are the standard modalities for diagnosis and screening of breast cancer. Mammography is the only screening imaging modality, which has been shown to improve the outcomes of patients with breast cancer (1). However, in women with dense breast tissues, the sensitivity of MMG decreases to 48 - 68% (2). Generally, breast US imaging is a complementary imaging tool for breast cancer diagnosis, which is particularly useful for women with a dense breast tissue (3). It is a non-invasive method, which does not require radiation and is effective in detecting early-stage breast cancer (4). Nevertheless, according to the American College of Radiology Imaging Network (ACRIN) protocol 6666, the application of breast US for breast cancer screening increases the false-positive rate (5). Therefore, it is associated with an increase in invasive procedures and short-term follow-up for benign breast lesions, which may lead to an increase in psychological and financial burdens on patients (6).
Breast-specific gamma imaging (BSGI) is a nuclear medicine breast imaging technique, which uses a physiological approach to identify lesions in the breast. Several studies have reported that BSGI improves the accuracy of breast cancer management (7). The benefits of BSGI include greater comfort for the patient, lower cost, and a less time-consuming procedure for physicians to interpret the results, although it involves the use of a radioactive tracer (8). The use of BSGI as a supplementary tool for breast cancer diagnosis has not been established yet. In the present study, it was hypothesized that the addition of BSGI to MMG or US, especially for patients with Breast Imaging-Reporting and Data System (BI-RADS) 0 or 4a lesions with a relatively low malignancy risk, could improve breast cancer diagnosis and reduce unnecessary biopsies of breast lesions, which require additional examinations or invasive procedures.
2. Objectives
This study aimed to determine whether the integration of BSGI into MMG or US interpretations could improve the diagnostic accuracy and reduce additional examinations or unnecessary biopsies for breast lesions.
3. Patients and Methods
3.1. Study Population
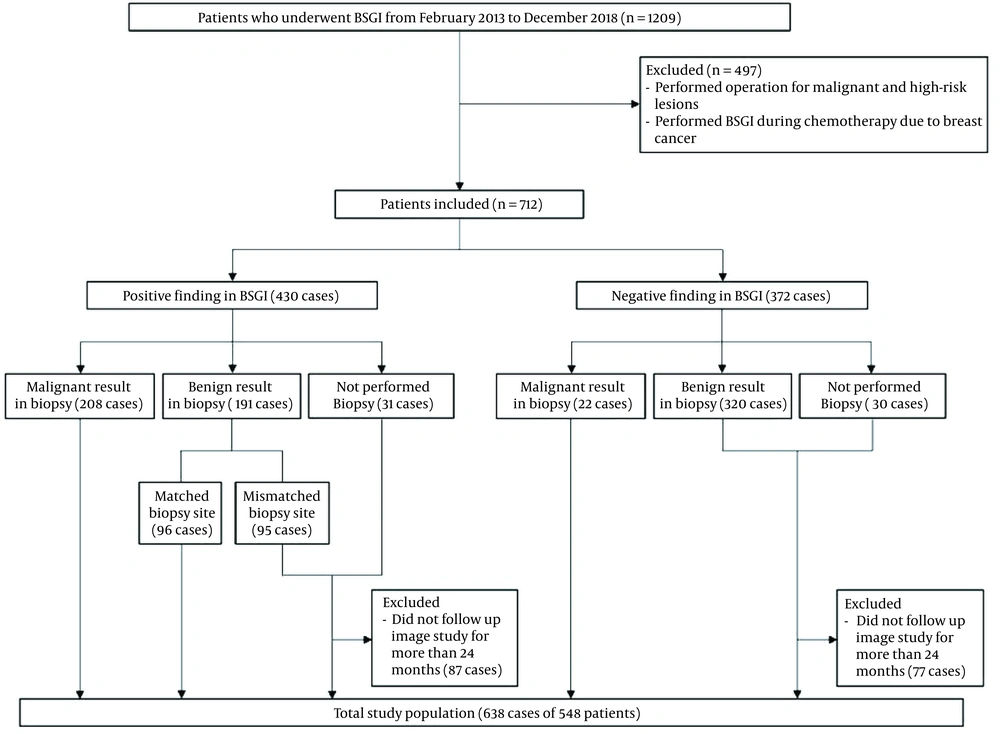
This retrospective study was approved by the institutional review board of Konyang University Hospital (IRB file No; KYUH 2021-05-011), and the requirement to obtain a written informed consent form for data access was waived. A total of 1209 patients who underwent BSGI from February 2013 to December 2018 were retrospectively reviewed. BSGI was performed for patients who were diagnosed with a malignant lesion before surgery, had suspicious lesions on US or MMG, or had multiple benign lesions. The process of patient enrollment is presented in Figure 1. Of 1209 patients, 661 were excluded from this study. In cases of multiple lesions on BSGI, each lesion was considered a single case. Also, in patients with lesions in both breasts, each lesion was considered as a single case.
3.2. Image Acquisition and Interpretation
3.2.1. MMG and Breast US Imaging
MMG was performed in the craniocaudal and mediolateral oblique projections (Lorad Selenia, Hologic, Marlborough, MA, USA). Breast US examinations were performed using an HDI 5000 scanner (Philips, Eindhoven, Netherlands) or an IU 22 scanner (Philips, Bothell, WA, USA) with a linear 10 - 12 MHz transducer. Nine patients with 10 lesions refused to undergo MMG. All images were interpreted by three breast radiologists with eight, nine, and 10 years of radiology experience, respectively. Any discrepancies were resolved through consensus. The MMG and breast US findings were evaluated based on the BI-RADS lexicon (9). Categories 0, 4, and 5 were considered positive; category 0 was considered positive, meaning that further examination of the lesion was required to exclude the possibility of malignancy; and categories 1, 2, and 3 were considered negative.
3.2.2. BSGI
BSGI was conducted after an intravenous injection of 30 mCi (1110 MBq) of technetium (99mTc)-sestamibi (Dong-A Pharmaceutical Co., Ltd, Seoul, Korea). Images were acquired immediately after the injection. The craniocaudal and mediolateral views were examined for each breast (approximately 10 minutes per view). Images were obtained with a high-resolution, breast-specific gamma camera with a small field of view (Dilon 6800 Gamma Camera, Dilon Technologies Inc., Newport News, VA, USA).
All BSGI images were interpreted by a nuclear medicine specialist, who was unaware of the pathology findings. The BSGI images with no focal lesion(s) or scattered physiological uptake were considered negative. The intensity of lesion uptake was categorized as follows: grade 1, mild (equal to or slightly greater than the subcutaneous fat); grade 2, moderate (more than mild, but less than twice as intense as the subcutaneous fat); and grade 3, marked or intense (at least twice as intense as the subcutaneous fat) (10). The BSGI images which showed a focal lesion with increased radiotracer uptake in the breast were considered positive. Lesions considered positive on BSGI were compared with those detected via other modalities (including MMG and US) to determine if they correspond to each other.
3.3. Histopathological Evaluation
Histopathological diagnoses were determined based on the electronic medical records of our institution. The final histopathological diagnoses were made based on the evaluation of surgical specimens. Core needle biopsy specimens were evaluated in patients who refused surgery or were transferred to other hospitals. Benign lesions were diagnosed based on the results of core needle biopsy or follow-up imaging of at least two years. High-risk lesions for which surgical excision was recommended, such as atypical ductal hyperplasia (ADH), atypical lobular hyperplasia (ALH), lobular carcinoma in situ (LCIS), intraductal papilloma, and mucocele-like lesions, were classified as intermediate (11).
In patients with malignant lesions, the histological type, including invasive ductal carcinoma (IDC), invasive lobular carcinoma (ILC), and other carcinomas (eg, metaplastic carcinoma), the presence of carcinoma in situ or extensive intraductal components, nuclear grade, estrogen receptor (ER) status, progesterone receptor (PR) status, HER2 expression, Ki-67 index, and tumor size were reviewed. Nuclear grade 3 was considered high, and grades 1 and 2 were regarded as low. The ER and PR were considered negative if their expression was less than 10% each; it was considered positive if their expression exceeded 10% each. The expression of HER2 gene was evaluated using the HercepTest (Dako, Glostrup, Denmark) and scored on a scale of 0 to 3+. Tumors with scores ≥ 3 or a ≥ 2.2-fold increase in HER2 gene amplification, as determined by fluorescence in situ hybridization, were considered positive for HER2 overexpression. Moreover, the Ki-67 index was considered positive if the Ki-67-positive nucleus content was 14% or greater. The tumor size was determined by the largest diameter.
3.4. Statistical Analysis
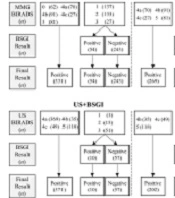
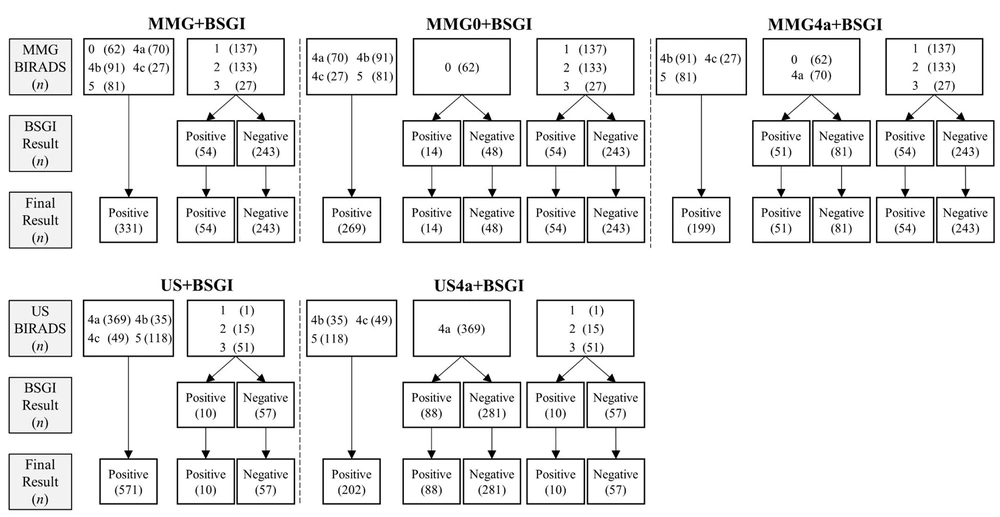
The diagnostic performance of BSGI, MMG, and US was compared for only malignant breast lesions and both malignant and intermediate lesions. The subgroups of US and MMG to which BSGI was added were divided. First, the group with both MMG and BSGI was divided into three subgroups: MMG+BSGI, MMG0+BSGI, and MMG4a+BSGI. In the MMG+BSGI group, if at least one of the MMG or BSGI results was positive, the result of the final assessment was considered positive for the MMG+BSGI group. The result of the final assessment of MMG+BSGI was considered negative only when both MMG and BSGI results were negative.
In the MMG0+BSGI group, the result of the final assessment was similar to that of MMG+BSGI, except for MMG category 0 lesions. For MMG category 0 lesions, the result of the final assessment was determined according to the BSGI results. If the BSGI result was negative, the result of the final assessment was considered negative, while if the BSGI result was positive, the result of the final assessment was considered positive. For example, if the MMG category of a lesion is 0 (positive), and the BSGI result is negative, the result of the final assessment is positive in the MMG+BSGI group, while it is negative in the MMG0+BSGI group.
In the MMG4a+BSGI group, if the MMG category was 0 or 4a, the result of the final assessment was determined according to the BSGI results. The breast US group was then divided into two subgroups: US+BSGI and US4a+BSGI. In the US+BSGI group, if either the breast US or BSGI result was positive, the result of the final assessment was considered positive. On the other hand, the final result was only considered negative when both US and BSGI results were negative. In the US4a+BSGI group, the final result was similar to that of US+BSGI, except for US category 4a lesions. For US category 4a lesions, the result of the final assessment was determined according to the result of BSGI. The evaluation methods used for each subgroup are shown in Figure 2.
The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated to detect only malignant lesions and both malignant and intermediate lesions by using each modality. The McNemar’s test was used to assess differences in the sensitivity and specificity of modalities. The receiver operating characteristic (ROC) curve analysis was performed, and the areas under the curve (AUCs) were compared between different modalities. Factors associated with false-positive and false-negative results were determined using Chi-square test or Fisher’s exact test. All statistical analyses were performed using MedCalc Version 19.6.4 (MedCalc, Mariakerke, Belgium) or SPSS version 19.0 (IBM Corp. Released 2010. IBM SPSS Statistics for Windows, Version 19.0. Armonk, NY: IBM Corp.). P-value less than 0.05 was considered statistically significant.
4. Results
A total of 548 patients (median age, 54.5 years; range: 25 - 89 years) with 638 breast lesions were enrolled in this study. Among 638 lesions, 230 (36.1%) were malignant, 60 (9.4%) were intermediate, and 348 (54.5%) were benign, as shown in Table 1. Nine patients did not undergo MMG, while all other patients underwent both MMG and BSGI. BSGI showed positive results for 278 lesions, including 203 malignant cases, five high-risk cases, and 14 cases of intraductal papilloma. BSGI showed negative results in 360 lesions, 292 of which were benign on biopsy or follow-up imaging studies. MMG showed positive results in 331 cases and negative results in 297 cases. Also, breast US showed positive results in 571 cases and negative results in 67 cases.
| Characteristics | No. of cases (%) |
|---|---|
| Age, years [range] | 54.5 [25 - 89] |
| Pathology of breast cancer (n = 230) | |
| Histological subtype | |
| IDC | 169 (73.5) |
| DCIS | 40 (17.4) |
| ILC | 9 (3.9) |
| Papillary carcinoma | 4 (1.7) |
| Metaplastic carcinoma | 2 (< 1) |
| Mucinous carcinoma | 2 (< 1) |
| ACC | |
| Othersa | |
| Tumor size | |
| < 1.0 cm | 42 (18.3) |
| ≥ 1.0 cm | 188 (81.7) |
| Nuclear grade | |
| Low | 132 (57.4) |
| High | 98 (42.6) |
| Estrogen receptor | |
| Positive | 175 (76.1) |
| Negative | 55 (23.9) |
| Progesterone receptor | |
| Positive | 152 (66.1) |
| Negative | 78 (33.9) |
| HER2 status | |
| Positive | 55 (23.9) |
| Negative | 175 (76.1) |
| Ki-67 index b | |
| Low | 37 (18.9) |
| High | 159 (81.1) |
Abbreviations: IDC, invasive ductal carcinoma; DCIS, ductal carcinoma in situ; ILC, invasive lobular carcinoma; ACC, adenoid cystic carcinoma.
a Other types include invasive carcinoma of no special type (n = 1), poorly differentiated carcinoma (n = 1), and Paget’s disease (n = 1).
b The Ki-67 index was reported for 196 cases.
4.1. Overall Diagnostic Performance of BSGI, MMG, and US for Detecting Malignant Lesions
The overall diagnostic performance of modalities for the detection of malignant lesions is shown in Table 2. For detecting malignant breast lesions, the sensitivity of BSGI (88.26%) was similar to that of MMG (87.95%) (P > 0.99). Although the sensitivity of BSGI was lower than that of US (97.83%), the specificity and AUC of BSGI (81.62% and 0.85, respectively) were significantly higher than those of MMG (66.83% and 0.77, respectively) and US (15.20% and 0.57, respectively) (P < 0.001).
| Malignant lesions | Benign and intermediate lesions | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) | AUC (95% CI) | |
|---|---|---|---|---|---|---|---|
| BSGI | 88.26 (83.38 - 92.12) | 81.62 (77.51 - 85.26) | 73.02 (68.69 - 76.95) | 92.50 (89.61 - 94.63) | 0.85 (0.82 - 0.88) | ||
| Positive | 203 | 75 | |||||
| Negative | 27 | 333 | |||||
| MMG b | 87.95 (82.95 - 91.90) | 66.83 (62.01 - 71.41) | 59.52 (55.94 - 63.00) | 90.91 (87.46 - 93.48) | 0.77 (0.74 - 0.81) | ||
| Positive | 197 | 134 | |||||
| Negative | 27 | 270 | |||||
| US | 97.83 (95.00 - 99.29) | 15.20 (11.85 - 19.05) | 39.40 (38.33 - 40.49) | 92.54 (83.49 - 96.82) | 0.57 (0.53 - 0.61) | ||
| Positive | 225 | 346 | |||||
| Negative | 5 | 62 | |||||
| MMG+BSGI | 96.43 (93.08 - 98.45) | 58.17 (53.19 - 63.03) | 56.10 (53.19 - 58.98) | 96.71 (93.67 - 98.31) | 0.77 (0.74 - 0.81) | ||
| Positive | 216 | 169 | |||||
| Negative | 8 | 235 | |||||
| MMG0+BSGI | 95.99 (92.51 - 98.15) | 69.80 (65.07 - 74.24) | 63.80 (60.25 - 67.20) | 96.91 (94.28 - 98.35) | 0.83 (0.80 - 0.86) | ||
| Positive | 215 | 122 | |||||
| Negative | 9 | 282 | |||||
| MMG4a+BSGI | 94.64 (90.83 - 97.20) | 77.23 (72.82 - 81.23) | 69.74 (65.76 - 73.44) | 96.30 (93.73 - 97.84) | 0.86 (0.83 - 0.89) | ||
| Positive | 212 | 92 | |||||
| Negative | 12 | 312 | |||||
| US+BSGI | 98.70 (96.24 - 99.73) | 13.24 (10.10 - 16.91) | 39.07 (38.11 - 40.04) | 94.74 (85.06 - 98.27) | 0.56 (0.52 - 0.60) | ||
| Positive | 227 | 354 | |||||
| Negative | 3 | 54 | |||||
| US4a+BSGI | 93.04 (88.95 - 95.97) | 78.92 (74.64 - 82.78) | 71.33 (67.27 - 75.08) | 95.27 (92.60 - 97.00) | 0.86 (0.83 - 0.89) | ||
| Positive | 214 | 86 | |||||
| Negative | 16 | 322 |
Abbreviations: BSGI, breast-specific gamma imaging; MMG, mammography; US, ultrasonography; CI, confidence interval; PPV, positive predictive value; NPV, negative predictive value; AUC, area under the curve.
a Values are expressed as %.
b Nine patients with 10 lesions did not undergo MMG because of patient’s refusal.
In the analysis of MMG subgroups where the BSGI results were added to the findings of MMG, the sensitivity of the subgroups (MMG+BSGI, 96.43%; MMG0+BSGI, 95.99%; and MMG4a+BSGI, 94.64%) was higher than that of MMG alone (P < 0.001, P < 0.001, and P = 0.003, respectively). However, their specificity was significantly higher than that of MMG only in the MMG4a+BSGI group (77.23%; P < 0.001). Moreover, in the subgroup analysis of US, the sensitivity of US4a+BSGI (93.04%) was significantly lower than that of US (P = 0.007). However, the specificity and AUC of US4a+BSGI (78.92% and 0.86, respectively) were significantly higher than those of US alone (P < 0.001 and P < 0.001, respectively).
4.2. Diagnostic Performance of Modalities for the Detection of Intermediate and Malignant Lesions
Intermediate lesions requiring surgical resection included 44 cases of intraductal papilloma and 16 high-risk lesions. They were clinically important lesions, and the diagnostic performance of imaging modalities was compared for their detection (Table 3). The specificity and AUC of BSGI (83.91% and 0.80, respectively) were significantly higher than those of MMG (67.92% and 0.73, respectively) and US (16.09% and 0.56, respectively) (P < 0.001). However, there was no significant difference in terms of sensitivity between BSGI (76.55%) and MMG (78.01%) (P = 0.79).
| Malignant and intermediate lesions | Benign lesions | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) | AUC (95% CI) | |
|---|---|---|---|---|---|---|---|
| BSGI | 76.55 (71.25 - 81.31) | 83.91 (79.62 - 87.61) | 79.86 (75.57 - 83.56) | 81.11 (77.63 - 84.16) | 0.80 (0.77 - 0.83) | ||
| Positive | 222 | 56 | |||||
| Negative | 68 | 292 | |||||
| MMG b | 78.01 (72.72 - 82.71) | 67.92 (62.72 - 72.81) | 66.47 (62.69 - 70.05) | 79.12 (75.04 - 82.69) | 0.73 (0.69 - 0.76) | ||
| Positive | 220 | 111 | |||||
| Negative | 62 | 235 | |||||
| US | 96.21 (93.31 - 98.09) | 16.09 (12.39 - 20.38) | 48.86 (47.58 - 50.15) | 83.58 (73.11 - 90.51) | 0.56 (0.52 - 0.60) | ||
| Positive | 279 | 292 | |||||
| Negative | 11 | 56 | |||||
| MMG+BSGI | 87.23 (82.77 - 90.90) | 59.83 (54.45 - 65.03) | 63.90 (60.70 - 66.97) | 85.19 (80.72 - 88.76) | 0.74 (0.70 - 0.77) | ||
| Positive | 246 | 139 | |||||
| Negative | 36 | 207 | |||||
| MMG0+BSGI | 85.46 (80.80 - 89.36) | 72.25 (67.21 - 76.91) | 71.51 (67.78 - 74.97) | 85.91 (82.02 - 89.07) | 0.79 (0.76 - 0.82) | ||
| Positive | 241 | 96 | |||||
| Negative | 41 | 250 | |||||
| MMG4a+BSGI | 83.33 (78.46 - 87.49) | 80.06 (75.45 - 84.14) | 77.30 (73.26 - 80.89) | 85.49 (81.87 - 88.49) | 0.82 (0.78 - 0.85) | ||
| Positive | 235 | 69 | |||||
| Negative | 47 | 277 | |||||
| US+BSGI | 97.24 (94.64 - 98.80) | 14.08 (10.60 - 18.18) | 48.54 (47.37 - 49.71) | 85.96 (74.68 - 92.71) | 0.56 (0.52 - 0.60) | ||
| Positive | 282 | 299 | |||||
| Negative | 8 | 49 | |||||
| US4a+BSGI | 81.32 (76.82 - 85.28) | 78.33 (74.25 - 81.93) | 83.73 (80.14 - 86.78) | 0.81 (0.78 - 0.84) | |||
| Positive | 235 | 65 | |||||
| Negative | 55 | 283 |
Abbreviations: BSGI, breast-specific gamma imaging; MMG, mammography; US, ultrasonography; CI, confidence interval; PPV, positive predictive value; NPV, negative predictive value; AUC, area under the curve.
a Values are expressed as %.
b Nine patients with 10 lesions did not undergo MMG because of patient’s refusal.
Table 3 presents a comparison of diagnostic performance for both intermediate and malignant lesions in the MMG subgroups. Among different subgroups, only the MMG4a+BSGI group showed significantly superior results to the group of MMG alone in terms of diagnostic performance (sensitivity, P = 0.02; specificity, P < 0.001; and AUC, P < 0.001). In the US subgroup analysis, similar to the comparison for detecting malignant lesions, lower sensitivity (81.03%) and higher specificity (81.32%) and AUC (0.81) values were found in the US4a+BSGI group compared to the group of US alone (P < 0.001).
4.3. Analysis of False-Positive and False-Negative Findings of BSGI
There were 75 BSGI false-positive findings, 72 of which also had pathological findings obtained via biopsy or surgery. Common pathological diagnoses were fibrocystic changes (18/72, 25.0%), fibroadenomas (18/72, 25.0%), and intraductal papilloma (14/72, 19.4%). Moreover, there were 26 BSGI false-negative results. The pathological results indicated IDC in 11 (42.3%) cases, including three cases associated with ductal carcinoma in situ (DCIS). There were 9 (34.6%) cases of DCIS, 4 (15.4%) cases of ILC, 1 (3.8%) case of invasive papillary carcinoma, and 1 (3.8%) case of mucinous carcinoma.
Factors associated with the false-negative results of BSGI were examined in this study. To investigate the association between tumor size and false-negative BSGI findings, the tumor size was divided into two groups: < 1.0 cm in diameter and ≥ 1.0 cm in diameter. Among 26 false-negative BSGI lesions, 13 (50%) were ≥ 1.0 cm in diameter, and 13 (50%) were < 1.0 cm in diameter. There was no significant association between false-negative BSGI findings and tumor size (P = 0.14). The Ki-67 index was reported for 14 out of 26 false-negative BSGI lesions. Lesions with a low Ki-67 index (8/14; 57.1%) accounted for a significantly higher proportion of false-negative interpretations compared to those with a high Ki-67 index (6/14; 42.9%) (P = 0.007). No other factors were associated with false-negative BSGI findings (nuclear grade, P = 0.54; ER status, P = 0.46; PR status, P = 0.43; and HER2 status, P = 0.41).
5. Discussion
BSGI showed sensitivity of 88.26% for breast cancer diagnosis, which is similar to the results of previous studies (89-96.4%) (12). The sensitivity of BSGI was comparable to that of MMG (87.95%) and lower than that of breast US (97.83%) in this study. In terms of specificity and AUC, BSGI (specificity, 81.62%; AUC, 0.85) was superior to MMG (specificity, 66.83%; AUC, 0.77) and US (specificity, 15.20%; AUC, 0.57). The diagnostic accuracy particularly improved when BSGI was additionally applied to evaluate MMG 0 and 4a lesions and US 4a lesions, which require an additional examination or tissue biopsy.
MMG is currently used as an effective screening tool for breast cancer detection. However, it has a limited predictive value, as well as false-positive rates (13). Several studies have reported that the addition of BSGI to MMG for women with a dense breast tissue increased the cancer detection rate (14). Regarding BI-RADS 0 or 4a lesions which required an additional examination or biopsy for diagnosis, the PPV was 6.8 - 7.2% for category 0 lesions and 2 - 10% for category 4a lesions (15), which are relatively low compared to the PPVs of category 4b (10 - 50%) and 4c (50 - 95%) lesions (9). To evaluate the usefulness of BSGI as a supplementary tool, a subgroup analysis of MMG was performed on BI-RADS 0 and 4a lesions. The sensitivity, PPV, and AUC of MMG0+BSGI and MMG4a+BSGI increased significantly compared to MMG for the detection of not only breast cancer, but also intermediate lesions. In the MMG4a+BSGI group, specificity also increased significantly. The PPV and specificity of MMG0+BSGI and MMG4a+BSGI were superior to those of MMG+BSGI for detecting not only breast cancer, but also intermediate lesions. The present results suggest that addition of BSGI to MMG, indicating category 0 or 4a lesions, may reduce the need for excessive follow-up studies or unnecessary biopsies.
The US examination is a complementary imaging tool, which is applied following MMG for breast cancer diagnosis. It is a noninvasive technique, which requires no radiation and is effective in detecting early-stage breast cancer. Therefore, it plays a substantial role in reducing the mortality rate of breast cancer. However, one disadvantage of using breast US is its high false-positive rate (6). In this study, the sensitivity of US was very high, whereas its specificity was low, as many biopsies were performed. To evaluate the usefulness of BSGI as a complementary tool to US, a subgroup analysis was performed by selective application of BSGI. The application of BSGI for US category 4a lesions significantly increased the specificity and AUC compared to US alone (Figure 3).
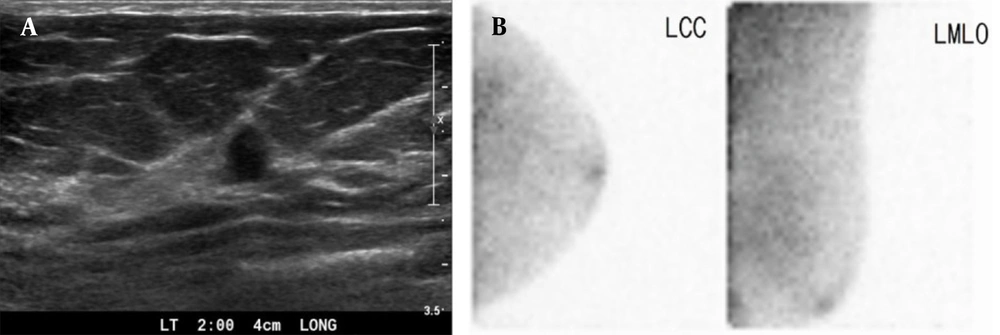
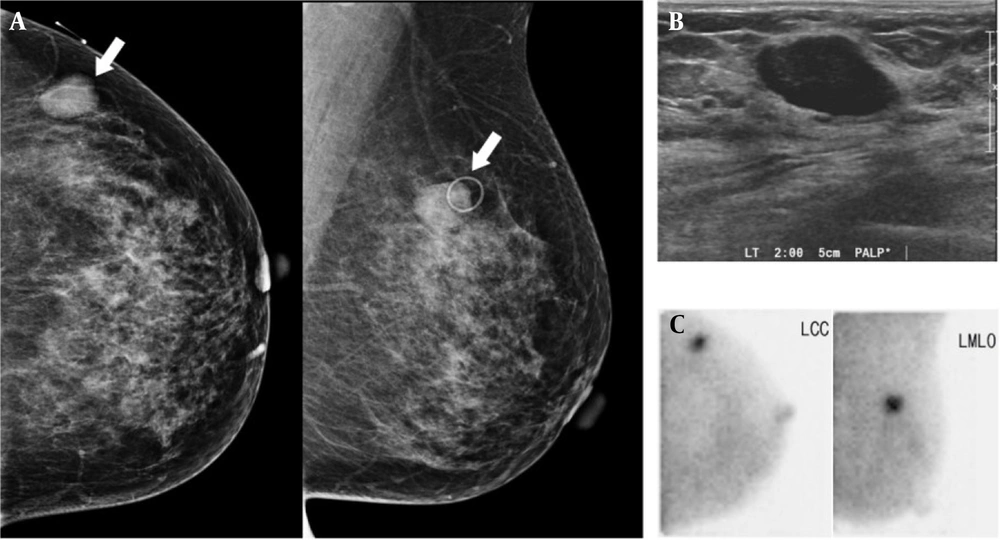
A 45-year-old woman with duct ectasia. Breast US. A, shows a small (7 mm) hypoechoic lesion with a taller-than-wide appearance in the left breast (BI-RADS 4a). B, BSGI indicates negative findings in the left breast (BSGI, breast-specific gamma imaging; US, ultrasonography; LCC, left craniocaudal; LMLO, left mediolateral oblique).
Similarly, for the detection of intermediate and malignant lesions, the specificity and AUC increased in subgroups which used BSGI for US category 4a lesions. In a study by Lee et al. (16), BSGI was used along with other imaging studies for patients with a BI-RADS 4a lesion, who either hesitated or refused biopsy. In patients with negative BSGI results, no malignancy was found in the imaging follow-up. Therefore, it was suggested that BSGI may be helpful for deciding on biopsy. Overall, the selective application of BSGI for patients with low-suspicion findings on US is expected to reduce unnecessary biopsies.
Among false-positive lesions, the most common pathological findings were fibrocystic change and fibroadenoma, which are benign proliferative diseases (Figure 4) and may be related to the principles of BSGI. Generally, BSGI is a nuclear medicine breast imaging tool using 99mTc-sestamibi, which accumulates in cells with increased blood flow and multiple mitochondria (17). Therefore, there is increased uptake not only in breast cancer cells, but also in benign proliferating cells. In other studies, fibrocystic change and fibroadenoma were the most common false-positive lesions (16, 18). Evidence suggests that some intraductal papillomas may have an abundant blood supply (18), which may explain the increased uptake of 99mTc-sestamibi in intraductal papilloma in this study.
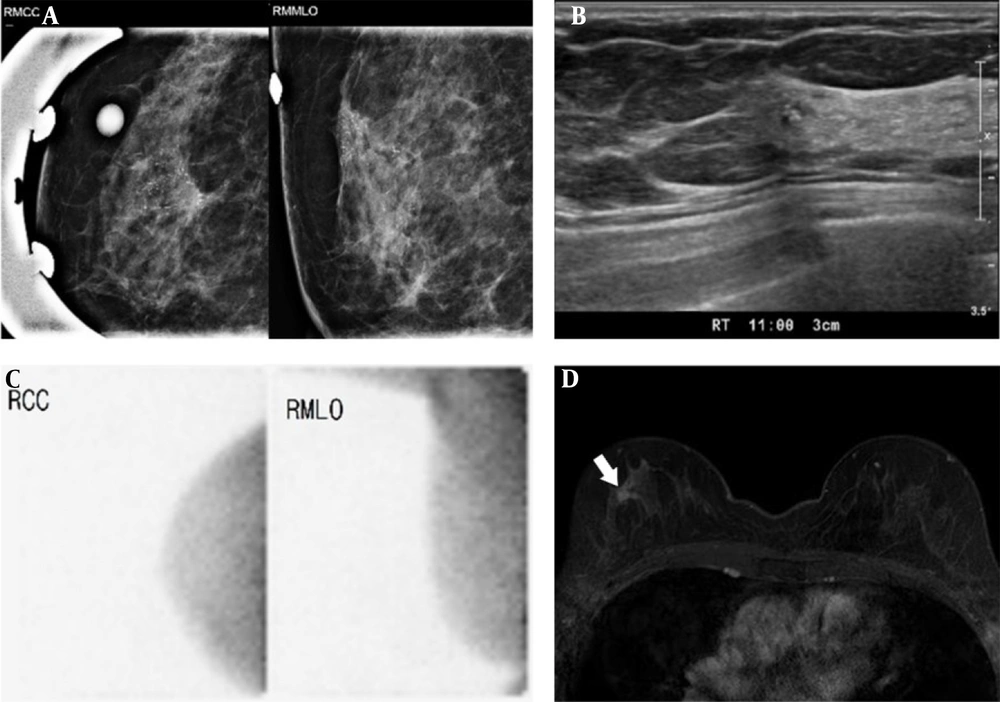
A 42-year-old woman with a palpable mass in the left breast, which was diagnosed as fibroadenoma after surgery. The left craniocaudal (CC) and mediolateral oblique (MLO) images. A, Show a circumscribed, oval-shaped, hyperdense mass (arrow) in the left upper outer quadrant. B, Breast US shows a circumscribed, oval-shaped, hypoechoic mass (2.3 cm) in the left breast (BI-RADS 4a). C, BSGI shows marked focal uptake in the left breast (BSGI, breast-specific gamma imaging; US, ultrasonography).
Regarding the false-negative lesions, several studies have shown that lesions with a diameter less than 1.0 cm are associated with false-negative BSGI findings, which may be attributed to the low cell count, low vascularity, and absence of inflammation in carcinomas (16, 19). However, in the present study, there was no significant association between the tumor size and false-negative BSGI findings (Figure 5). Besides, the nuclear grade, ER status, PR status, and HER2 expression were not significantly associated with the false-negative BSGI findings. However, a significant association was found between a low Ki-67 index and false-negative BSGI findings in this study. Generally, the Ki-67 is a protein found in all proliferating cells (20); a lower Ki-67 index indicates a lower degree of cell proliferation. Therefore, although the lesion is malignant, it can be assumed that BSGI will yield a negative result, as the Ki-67 index is low for the lesion. In other studies (19), there was no significant association between the Ki-67 index and false-negative BSGI findings, and further relevant studies are needed.
DCIS of the right breast in a 62-year-old woman. The right craniocaudal (CC) and mediolateral oblique (MLO) images. A, Show regionally distributed, fine pleomorphic, and linear branching microcalcifications in the right upper outer breast (BI-RADS 4c). Breast US (B) shows ill-defined, hypoechoic parenchymal changes with echogenic dots in the right breast at the corresponding location on MMG (BI-RADS 4c). However, BSGI (C) shows no focal uptake in the right breast. The breast MR image (D) shows an enhancing mass (1.9 × 1.1 × 1.9 cm) (arrow) in the right breast (DCIS, ductal carcinoma in situ; BSGI, breast-specific gamma imaging; US, ultrasonography; MMG, mammography).
Since BSGI uses radioactive isotopes, there is always a concern of whole-body radiation exposure. Hendrick and Tredennick (21) studied the benefit-to-risk ratio of radiation in MMG, BSGI, and MMG plus BSGI for breast cancer screening by age group. In screening tests, including BSGI, the benefit-to-risk ratio tended to increase less, as radiation exposure was greater than that of MMG alone. However, Hruska reported that the likelihood of carcinogenesis in the range of radiation doses typically used for medical imaging (effective dose, < 25 mSv) is very low and may not exist (22). Even with a low dose of 99mTc-sestamibi (7 - 10 mCi), there were no significant differences in terms of diagnostic performance, including sensitivity and specificity (23). Therefore, low-dose BSGI can reduce radiation exposure and help diagnose breast cancer.
The present study had the advantage of including a large number of patients who underwent BSGI and had histopathological results. The application of BSGI for BIRADS 0 or 4a lesions on MMG and category 4a lesions on US improved the diagnostic accuracy. Also, unnecessary biopsy recommendations could be reduced by applying BSGI.
There were some limitations to the present study. First, there is a possibility of selection bias, considering the retrospective design of this study. Second, it is difficult to generalize the results of this study because it involved a single institutional review. Third, the time for determining the benignity of a lesion was set at two years; consequently, lesions that did not change on MMG or US for two years were considered benign. Since not all patients underwent biopsy and pathological examinations, some of these lesions might have been proven to be malignant in further follow-ups.
In conclusion, based on the present findings, BSGI plus MMG or US could improve the diagnostic performance for detecting breast cancer, especially BI-RADS 0 and 4a lesions. Additionally, it could reduce the need for further examinations or unnecessary biopsies.