1. Background
The immaturity of lung development and immune function in infants increases the incidence of respiratory diseases. Besides, rapid development of CT screening technology has led to an increase in the frequency of CT examinations in infants. Nevertheless, in the rapid growth period, infants are more sensitive to radiation than adults, with an increased risk of teratogenicity and carcinogenicity. In this regard, a previous study found a significant relationship between X-ray radiation dose from CT scans and the risk of brain tumors (1). Therefore, in imaging examinations of infants, we should shift focus from clear visualization of lesions to minimization of radiation dose while meeting the diagnostic requirements (2).
With an increase in the frequency of CT examinations of infants, radiation dose has become a great concern. The current methods of radiation dose reduction mostly employ automatic tube current modulation techniques (3, 4). However, the lung radiation dose increases by increasing the attenuation coefficient due to the difference in tissue development level when using the automatic tube current modulation technique for infants aged 0 - 3 years. Here, the question arises as to whether there is a method that employs lower radiation doses than automatic tube current modulation. Therefore, the present study aimed to investigate the feasibility of a fixed ultralow tube current combined with the iDose4 iterative reconstruction technique for low-dose scanning of the chest in infants (at a tube voltage of 80 kV) and to compare it with the automatic tube current modulation technique.
2. Objectives
The present study aimed to explore the feasibility of a fixed ultralow tube current combined with the iDose4 iterative reconstruction technique at a low tube voltage (80 kV) in low-dose CT imaging of infant lungs.
3. Patients and Methods
3.1. Ethical Statement
This study was approved by the Institutional Review Board of Children's Hospital of Chongqing Medical University, China (NO.2019-145; link: https://figshare.com/s/881564130d0520144e53). The consent of children's guardians was also obtained, and they signed an informed consent form.
3.2. Participants
A total of 90 infants, aged 0 - 3 years, who underwent both chest plain CT scan and arterial scan due to a medical condition, were randomly included from January to December 2019 in the experimental group, and only the data of chest plain CT were collected for the experimental group. Besides, 90 infants, aged 0 - 3 years, who underwent chest plain CT scan, were randomly allocated to the control group. Both the experimental and control groups were divided into three subgroups according to age: 0-1-year-old group, 1-2-year-old group, and 2-3-year-old group (30 patients per group). It should be noted that the developmental status of infants under the age of three years varies greatly. Differences in height and weight can lead to differences in X-ray absorption and image quality. To reduce the effect of body size on the experimental data, they were grouped by age, and the radiation dose and image quality were compared in the same age subgroup.
The enhanced plain CT scan phase was performed for the experimental group at 80 kV under a fixed tube current (30 mA), while the arterial phase of enhanced CT examination was performed under conventional conditions (80 kV) with automatic tube current modulation (30 - 150 mA). On the other hand, for the control group, plain CT scan was performed at 80 kV with automatic tube current modulation (30 - 150 mA) under conventional conditions. The dose right index (DRI) was eight for the 0-1-year-old subgroup and nine for the 1-3-year-old subgroup (3, 5).
The inclusion criteria were as follows: (1) 5% < body mass index (BMI) < 95%; (2) no massive pneumothorax on imaging, no pleural effusion, no lung consolidation, no large tumors, no severe thoracic deformities, no severe cardiac abnormalities, and no hepatic or renal insufficiency; (3) no allergy to iodine; and (4) lack of enhanced CT scan or iodine contrast injection within 14 days.
Regarding the sedation method, infants who could not cooperate with chest CT imaging were examined after sedation in the sedation center of our hospital. For sedation, intranasal dexmedetomidine (3 µg/kg) and oral chloral hydrate (40 mg/kg) were administered. Contrast enhancement was performed via contrast injection into the right elbow vein.
3.3. CT Scan Technique
In this study, a Philips 256-Layer Brilliance iCT Scanner (Royal Philips; Amsterdam, Netherlands), an injector system (Bracco Injeneering S.A., Empower CTA, Milan, Italy), and an Advantage Workstation (AW4.6, GE Healthcare, Waukesha, Wisconsin, USA) were used. Regarding the scan parameters, all infants were placed in the supine position, with their feet advanced and both upper limbs held straight up next to the ears. The scans were acquired from the tip of the lung to the base of the diaphragm. The following parameters were applied: Collimation, 128 × 0.625 mm; rotation speed, 0.5 s/r; pitch, 1; thickness, 5 mm; reconstruction thickness, 1 mm; and matrix size, 512 × 512.
All images were reconstructed using the iDose4 technology on the lung window. The reconstruction level was three for the 0-1-year-old subgroup and four for the 1-2-year-old and 2-3-year-old subgroups. Generally, the Recon mode in reconstruction has 1 - 7 levels. The image noise decreases with an increase in the reconstruction level as the signal-to-noise ratio (SNR) and image quality increase. However, after reaching the optimal reconstruction level, the image clarity decreases by increasing the reconstruction level, and the image edges become blurred. Besides, reconstruction levels at different ages have different effects on the image quality (3, 5).
3.4. Radiation Dose
The volume CT dose index (CTDIvol) and dose-length product (DLP) were automatically generated by a computer, and the effective dose (ED) was calculated as follows:
ED (mSv)= k×DLP
where k is 0.039 mSv/mGy∙cm for the 0-1-year-old subgroup and 0.026 mSv/mGy∙cm for the 1-3-year-old subgroup (6, 7).
3.5. Image Analysis
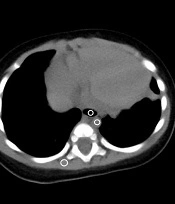
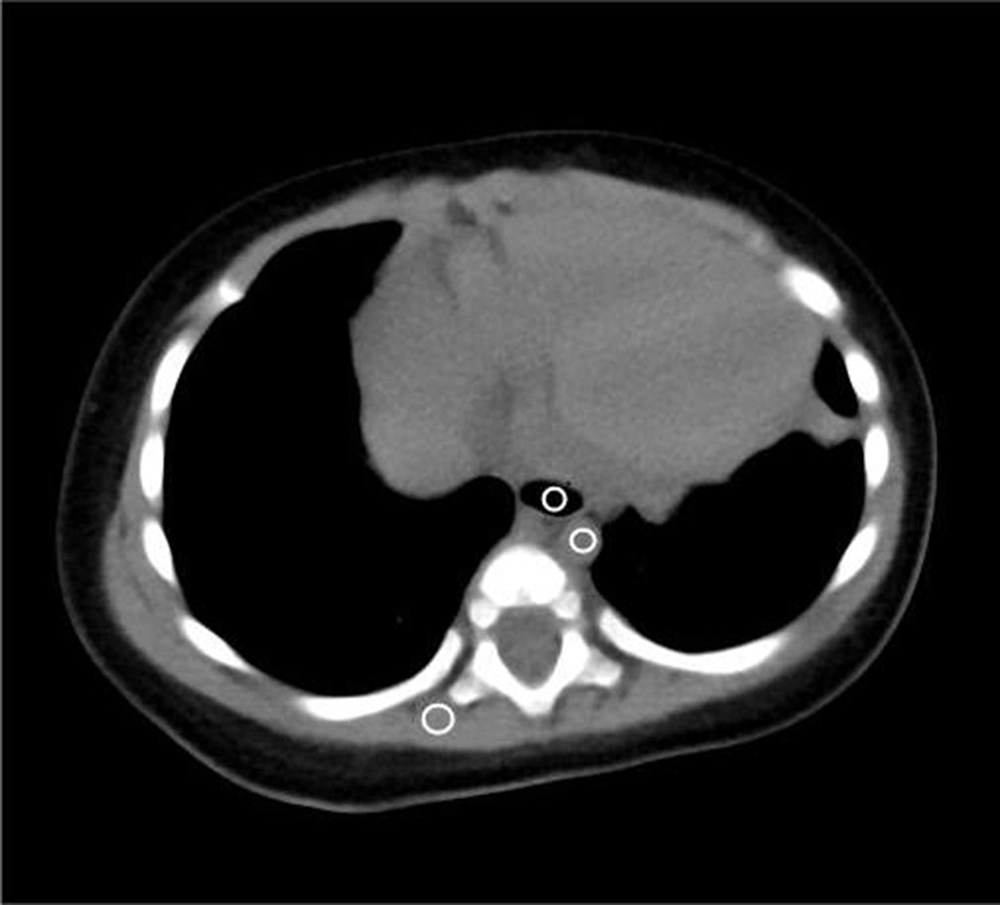
The collected data were imported into a GEAW 4.6 Workstation. For each group, the image noise was quantitatively evaluated by measuring the standard deviation (SD) of Hounsfield units and SNR. The CT values for the erector spinae region of interest (ROI) (30 ± 2 mm²), tracheal ROI (15 ± 2 mm²), descending aorta ROI (10 ± 2 mm²), and SD of image noise (SD of pixel values from uniform image regions) were measured at the level of the trachea carina in the mediastinal window by three technologists with five years of experience (or more) by calculating the ROI. The ROI was measured three times in the same position, and the average value was calculated. The SD of image noise and SNR were calculated for each tissue by considering the erector spinae SD as background noise (SNR = CT value/SD erector spinae) (8, 9) (Figure 1).
The subjective quality of images (lung window width, 1400 HU; window level, -450 HU) was evaluated by two thoracic radiologists with five years of experience, without any information on the infants or scanning parameters. The subjective image quality was evaluated on a five-point scale, with a score ≥ 3 satisfying the diagnostic requirements and indicating a successful examination. The scale was as follows: Score 5, clear anatomical structures and lesions and good contrast without artifact or noise; score 4, anatomical structures and lesions with few artifacts or noise; score 3, adequate image quality for interpretation with mild artifacts or noise; score 2, partial impairment of image quality for diagnostic purposes duo to severe artifact or noise; and score 1, marked impairment of image quality for diagnostic purposes with severe artifact or noise (10-12).
3.6. Statistical Analysis
IBM SPSS version 25.0 (released in 2017, IBM SPSS Statistics for Windows, IBM Corp., Armonk, NY, USA) was used for data analysis. The measurements are expressed as mean and SD. Chi-square test was used for sex distribution. Differences in BMI, CTDIvol, DLP, ED, SNR, and subjective image scores were compared between the experimental and control groups in similar age subgroups by two-sample independent t-test. The level of statistical significance was set at P < 0.05. The consistency of subjective image quality scores by the two diagnosticians was examined by kappa coefficient test: Kappa coefficient ≥ 0.75, good agreement; 0.75 > kappa coefficient ≥ 0.4, moderate agreement; and kappa coefficient < 0.4, poor agreement.
4. Results
4.1. General Information of the Participants
The 0-1-year-old subgroup included 17 males and 13 females in the experimental group (age: 0.40 ± 0.22 years; BMI: 16.71 ± 1.78 kg/m2) and 16 males and 14 females in the control group (age: 0.41 ± 0.25 years, BMI: 17.54 ± 1.52 kg/m2). Also, the 1-2-year-old subgroup included 16 males and 14 females in the experimental group (age: 1.5 ± 0.26 years, BMI: 16.38 ± 1.42 kg/m2) and 15 males and 15 females in the control group (age: 1.39 ± 0.25 years, BMI: 16.49 ± 1.37 kg/m2). Moreover, the 2-3-year-old subgroup included 14 males and 16 females in the experimental group (age: 2.52 ± 0.27 years, BMI: 15.36 ± 1.10 kg/m2) and 14 males and 16 females in the control group (age: 2.4 ± 0.24 years, BMI: 15.86 ± 1.30 kg/m2). There were no significant differences in terms of age, sex, and BMI in similar age subgroups of the experimental and control groups (P > 0.05) (Table 1).
| Variables | Age subgroup | ||
|---|---|---|---|
| 0 - 1 years old | 1 - 2 years old | 1 - 3 years old | |
| Male-to-female ratio | |||
| Experimental group | 17:13 | 16:14 | 14:16 |
| Control group | 16:14 | 15:15 | 14:16 |
| χ2 value | 0.067 | 0.067 | 0.000 |
| P-value | 0.795 | 0.796 | 1.000 |
| BMI (kg/m2) | |||
| Experimental group | 16.71 ± 1.78 | 16.38 ± 1.42 | 15.36 ± 1.10 |
| Control group | 17.54 ± 1.52 | 16.49 ± 1.37 | 15.86 ± 1.30 |
| T-value | -1.944 | -0.321 | -1.582 |
| P-value | 0.057 | 0.749 | 0.119 |
| Sedation requirement | |||
| Experimental group | 30 | 30 | 30 |
| Control group | 30 | 30 | 30 |
Abbreviation: BMI, body mass index.
4.2. Radiation Doses
In the 0-1-year-old subgroup, the mean CTDIvol, DLP, and ED were 0.71 mGy, 18.52 mGy/cm, and 0.48 mSv in the experimental group and 0.87 mGy, 23.35 mGy/cm, and 0.91 mSv in the control group, respectively. In the 2-3-year-old subgroup, the mean CTDIvol, DLP, and ED were 0.66 mGy, 19.24 mGy/cm, and 0.5 mSv in the experimental group and 1.03 mGy, 28.72 mGy/cm, and 0.75 mSv in the control group, respectively. Moreover, in the 2-3-year-old subgroup, the mean CTDIvol, DLP, and ED were 0.60 mGy, 17.63 mGy/cm, and 0.46 mSv in the experimental group and 1.07 mGy, 30.31 mGy/cm, and 0.79 mSv in the control group, respectively.
The CTDIvol, DLP, and ED showed significant differences between the experimental and control groups in the same age subgroup (P < 0.05), and the corresponding values were significantly lower in the experimental group compared to the control group. In the experimental group, as compared to the control group, the ED decreased by 47.25% in the 0-1-year-old subgroup, by 33.33% in the 1-2-year-old subgroup, and by 41.77% in the 2-3-year-old subgroup (Table 2).
| Groups | Age range of 0 - 1 years | Age range of 1 - 2 years | Age range of 2 - 3 years | ||||||
|---|---|---|---|---|---|---|---|---|---|
| CTDIvol (mGy) | DLP (mGy/cm) | ED (mSv) | CTDIvol (mGy) | DLP (mGy/cm) | ED (mSv) | CTDIvol (mGy) | DLP (mGy/cm) | ED (mSv) | |
| Experimental group | 0.71 ± 0.04 | 18.52 ± 1.95 | 0.48 ± 0.05 | 0.66 ± 0.50 | 19.24 ± 1.88 | 0.50 ± 0.01 | 0.60 ± 0.00 | 17.63 ± 1.78 | 0.46 ± 0.05 |
| Control group | 0.87 ± 0.14 | 23.35 ± 4.89 | 0.91 ± 0.19 | 1.03 ± 0.16 | 28.72 ± 4.01 | 0.75 ± 0.10 | 1.07 ± 0.08 | 30.31 ± 4.33 | 0.79 ± 0.11 |
| T-value | -6.127 | -5.028 | -11.917 | -12.386 | -11.716 | -11.716 | -29.357 | -14.832 | -14.832 |
| P-value | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
Abbreviations: CTDIvol, volume CT dose index; DLP, dose-length product; ED, effective dose.
a Data are presented as mean ± SD (n = 30).
4.3. Objective Image Quality
In the 0-1-year-old subgroup, the SNR of the erector spinae was significantly lower in the experimental group compared to the control group (P < 0.05), while the SNR of the trachea and descending aorta was not significantly different (P > 0.05). In the 1-2-year-old subgroup, the SNR of the erector spinae and descending aorta was significantly lower in the experimental group compared to the control group (P < 0.05), whereas the difference in the SNR of the trachea between the groups was not significant (P > 0.05). In the 2-3-year-old group, the SNRs of the erector spinae, trachea, and descending aorta were all significantly lower in the experimental group compared to the control group (P < 0.05) (Table 3).
| Groups | Age range of 0 - 1 years | Age range of 1 - 2 years | Age range of 2 - 3 years | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Erector spinae | Trachea | Descending aorta | Erector spinae | Trachea | Descending aorta | Erector spinae | Trachea | Descending aorta | |
| Experimental group | 4.33 ± 1.20 | -54.9 ± 12.32 | 3.33 ± 0.89 | 4.36 ± 1.28 | -55.96 ± 20.06 | 3.00 ± 0.89 | 4.18 ± 0.71 | -58.36 ± 10.33 | 2.82 ± 0.71 |
| Control group | 5.08 ± 1.51 | -62.56 ± 17.41 | 3.88 ± 1.38 | 5.67 ± 1.50 | -112.11 ± 207.02 | 4.05 ± 1.00 | 5.16 ± 1.10 | -74.78 ± 20.26 | 3.42 ± 1.01 |
| T-value | -2.113 | 1.967 | -1.848 | -3.663 | 1.503 | -4.341 | -4.098 | 3.953 | -2.622 |
| P-value | 0.039 | 0.054 | 0.070 | 0.001 | 0.138 | <0.001 | <0.001 | <0.001 | 0.011 |
a Data are presented as mean ± SD (n = 30).
4.4. Subjective Evaluation
There was a good level of agreement between the assessments performed by the two diagnosticians (A and B) (Table 4).
| Physician A | Physician B | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Score 5 | Score 4 | Score 3 | Score 2 | Score 1 | Score 5 | Score 4 | Score 3 | Score 2 | Score 1 | Kappa value | |
| 0 - 1 years old | |||||||||||
| Experimental group | 25 | 4 | 1 | 24 | 3 | 3 | 0.684 | ||||
| Control group | 26 | 4 | 25 | 5 | 0.870 | ||||||
| 1 - 2 years old | |||||||||||
| Experimental group | 24 | 4 | 2 | 24 | 4 | 2 | 1.000 | ||||
| Control group | 27 | 3 | 26 | 4 | 0.839 | ||||||
| 2 - 3 years old | |||||||||||
| Experimental group | 26 | 4 | 25 | 5 | 0.890 | ||||||
| Control group | 30 | 30 | 1.000 | ||||||||
There were no significant differences in the subjective image quality scores between the experimental and control groups in similar age subgroups (P > 0.05) (Table 5).
| 0 - 1 years old | 1 - 2 years old | 2 - 3 years old | ||||
|---|---|---|---|---|---|---|
| Experimental group | Control group | Experimental group | Control group | Experimental group | Control group | |
| Physician A | 4.8 ± 0.48 | 4.87 ± 0.35 | 4.73 ± 0.58 | 4.9 ± 0.30 | 4.87 ± 0.35 | 5 ± 0.00 |
| Physician B | 4.7 ± 0.65 | 4.83 ± 0.38 | 4.73 ± 0.58 | 4.87 ± 0.35 | 4.83 ± 0.38 | 5 ± 0.00 |
| T-value | 0.675 | 0.356 | 0 | 0.396 | 0.356 | |
| P-value | 0.502 | 0.723 | 1 | 0.694 | 0.723 | |
a Data are described as mean ± SD (n = 30).
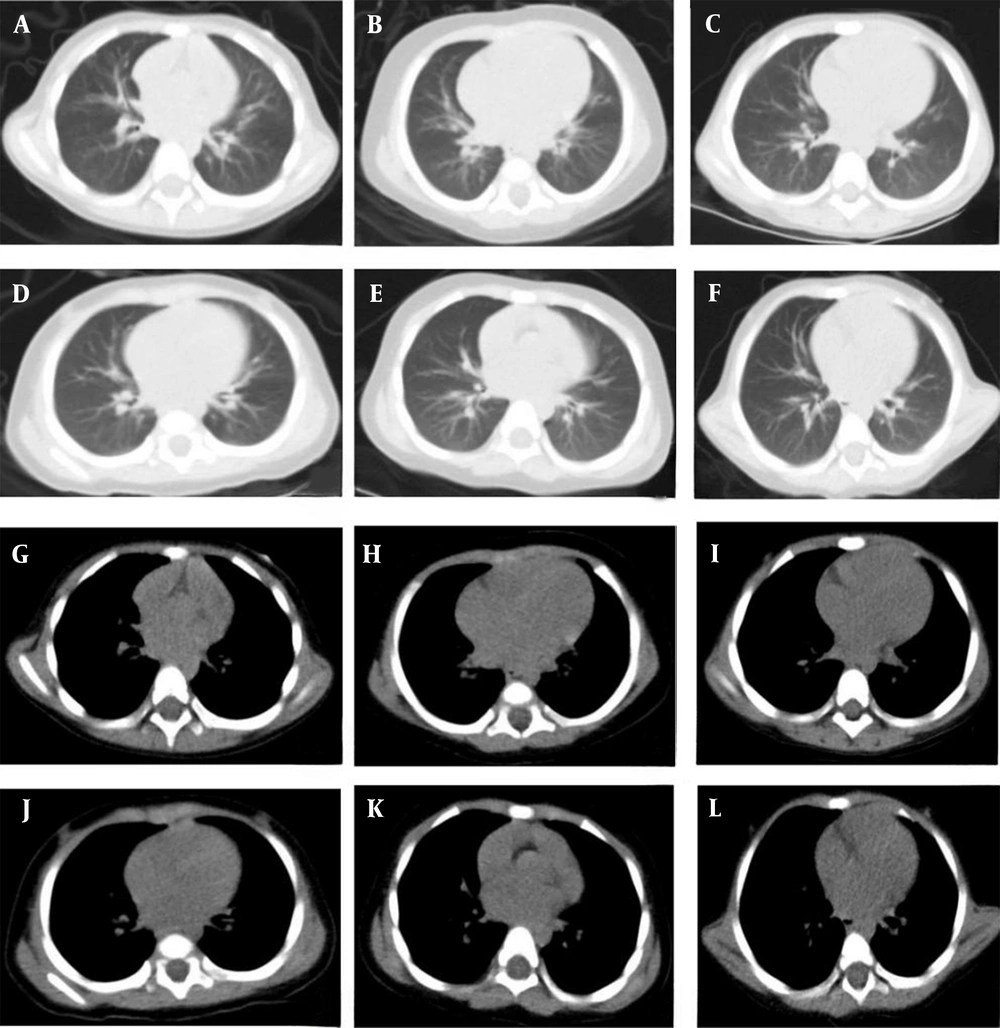
The images reconstructed with the iDose4 reconstruction technique in different age subgroups of the experimental and control groups are shown in Figure 2.
A, B, and C show the lung windows for the age subgroups of 0 - 1 years, 1 - 2 years, and 2 - 3 years in the control group; D, E, and F represent the lung windows for the age subgroups of 0 - 1 years, 1 - 2 years, and 2 - 3 years in the experimental group; G, H, and I represent the mediastinal windows for the age subgroups of 0 - 1 years, 1 - 2 years, and 2 - 3 years in the control group; J, K, L represent the mediastinal windows for the age subgroups of 0 - 1 years, 1 - 2 years, and 2 - 3 years in the experimental group.
5. Discussion
The anatomical structure of the chest, which consists of air-containing alveoli, muscles, soft tissues, and bones (with major variations in tissue density), makes low-dose CT possible. The X-ray exposure dose is proportional to the square of tube voltage; therefore, reducing the tube voltage can significantly decrease the radiation dose. In a previous study, the radiation dose was reduced by 37% at 100 kV compared to 120 kV (13). Additionally, Pan et al. (14) and Meng et al. (15) found that reducing the tube voltage could significantly reduce the radiation dose. However, reduction of tube voltage led to a decrease in direct photon flow, which had a direct effect on image noise and streak artifacts and could influence the diagnostic value of the image (16). To improve the image quality, the tube current needs to be increased; therefore, it is not advisable to simply reduce the tube voltage to decrease the radiation dose.
The radiation dose has a linear relationship with the tube current. Reduction of tube current can effectively decrease the radiation dose. In a study by Zhang et al. (17), the radiation dose was reduced by 87.6%, 75.2%, and 62.8% at 20, 40, and 60 mA, respectively compared to 100 mA; simultaneously, the image noise increased, while the SNR reduced (18). In another study, the radiation dose nearly doubled when the tube current was reduced from 200 mA to 50 mA (19); therefore, reduction of radiation dose cannot be achieved by simply decreasing the tube current. Generally, it is an important and arduous task to reach the best combination of tube voltage and tube current while making a diagnosis.
The ASIR technology selectively removes noise by improving the original data reconstruction algorithm, thereby ensuring image quality when scanning at lower doses (20-23). The Philips introduced iDose4, a fourth-generation iterative reconstruction technique based on a dual-space multi-model, which can effectively improve the image spatial resolution and density resolution, remove noise and suppress low-dose artifacts, and sustain the fidelity of CT images by maintaining the structural information through the frequency noise spectrum. Some studies have reported that iDose4 iterative reconstruction can be applied to low-dose CT scans (24-27). The radiology branch of the Chinese Medical Association recommends that the scanning conditions for low-dose CT (LDCT) should be 100 - 120 kV and < 30 mA, using the new generation of nonlinear iterative reconstruction algorithms. In this study, a Philips 256-Layer Brilliance iCT Scanner was used, and a fixed tube current of 30 mA combined with the iDose4 iterative reconstruction technique at a low tube voltage (80 kV) was considered as the low-dose scanning parameter.
The thymus gland is found at the upper end of the sternum between the right and left lung lobes, below the thyroid gland. The transverse diameter of the thymus is greater than its long diameter during fetal life and becomes narrower and thicker after birth due to pressure on the chest cavity. It is relatively heavy at birth and continues to develop with age until it gradually deteriorates during adolescence (28-30). Because of the presence of thymic structures, at a low tube voltage of 80 kV, when the control group used automatic tube current modulation, the radiation dose increased due to the increased attenuation of localized image to X-rays, and the equipment automatically increased the tube current to ensure image quality.
In the present study, a fixed tube current of 30 mA was used for the experimental group, which did not change with the attenuation coefficient. Combined with the iDose4 iterative reconstruction technique, the radiation dose was maintained at a lower level, and the image quality was improved without affecting the diagnostic accuracy. Accordingly, the radiation dose was lower in the experimental group compared to the control group in all age subgroups. The 0-1-year-old infants showed lower lung inflation compared to older infants and had the largest relative thymus weight and attenuation coefficient. Therefore, the 0-1-year-old subgroup exhibited the most significant ED reduction in the experimental group compared to the control group.
To ensure the stability of data, the CT values of the erector spinae, trachea, and descending aorta were measured at the level of the tracheal ramus for each child (31). In this study, there was a significant difference in the SNR of the erector spinae in the 0-1-year-old subgroup. The SNR was significantly higher in the control group compared to the experimental group, while the SNR of the descending aorta and trachea showed no significant differences. The SNRs of the erector spinae and descending aorta were significantly different in the 1-2-year-old subgroup; the SNR of the control group was significantly higher than that of the experimental group, while the SNR of the trachea showed no significant differences. There were significant differences in the SNR of the erector spinae, trachea, and descending aorta in the 2-3-year-old subgroup; the SNR was significantly higher in the control group compared to the experimental group. According to this finding, the image noise increased as the radiation dose decreased. With advancing age, the difference in the image SNR of each tissue gradually became significant, while the difference in the trachea image noise was not significant in the 0-2-year-old subgroup, thereby ensuring the image quality for lung tissues containing air. Although the image noise increased in the 2-3-year-old subgroup, the difference in subjective image quality scores was not significant between the experimental and control groups and did not affect the diagnostic accuracy (32).
The participants of this study were divided into three age subgroups considering the differences in their body size at different ages. According to the literature, the corresponding lowest dose index (DRI) was selected for each age subgroup to make the experiment more scientific, reasonable, and practical. In this study, for the experimental group, the flat-scan phase was used in enhanced CT imaging, even if the image did not meet the diagnostic requirements due to dose reduction; also, in the enhanced phase, conventional scanning conditions were used to ensure diagnosis. In this study, the CTDIvol and DLP, automatically generated by the computer, were collected to calculate the ED as a radiation dose parameter. The ED responds to non-uniform radiation doses received by different parts of the body and is derived from the weighting factor K according to different ages in different parts of the body to ensure the scientific accuracy of data.
The present study had some shortcomings. First, it mainly evaluated the lung tissue structure and lesions, without evaluating structures, such as the mediastinum and bone tissue. Second, the sample size was small, and a larger population is needed to confirm the results. Third, only infants and children aged 0 - 3 years were included in this study, and no other age subgroups were examined; therefore, other age subgroups can be added to validate the results. Finally, this study only included a low-dose plain phase and did not include a booster phase.
In conclusion, a fixed low tube current (30 mA) at a low tube voltage (80 kV), combined with the iDose4 iterative reconstruction technique, for lung CT scans in infants and children aged 0 - 3 years could reduce the radiation dose, while meeting the diagnostic requirements.