1. Background
Malignant tumors are a major cause of mortality worldwide, posing a serious threat to human health. According to the Global Cancer Epidemiology Database (GLOBOCAN), in 2018, China recorded 4.3 million new cancer cases and 2.9 million cancer-related deaths (1-3). During tumor development, malignant cells detach from the original tumor site and spread to other parts of the body through a complex physiological process known as metastasis. Metastasis, which finally leads to death by gradually damaging the normal function of organs, has been the main cause of mortality in patients with malignant tumors, such as breast cancer, prostate cancer, lung cancer, kidney cancer, thyroid cancer, and gastrointestinal and reproductive system tumors (4-7). The incidence of bone metastasis in cancer patients is witnessing a rapid increase. It is now identified as the third most common site for malignant tumor metastasis, following metastasis to the lungs and liver (8). Nearly 20% (9) of malignant tumor patients exhibit the symptoms of bone metastasis. The overall incidence of bone metastasis is estimated to be 32.5%, which is 35 to 40 times higher than that of primary malignant bone tumors (10, 11).
Currently, the exact molecular mechanism underlying bone metastasis remains unclear. The goal of therapy for bone metastasis is to halt tumor progression and alleviate the symptoms. While numerous surgical treatments have been developed to manage bone metastases, aiming to alleviate clinical symptoms and improve bodily function, their therapeutic efficacy in treating bone metastases remains limited. Meanwhile, surgical treatment carries a number of potential risks, including significant trauma, substantial blood loss, particularly in cases with excessive blood vessels, and pathological fractures or soft tissue swelling (12, 13). Therefore, a safe and effective treatment method is needed to relieve the pain of patients with bone metastasis more effectively, improve the patients’ quality of life, and increase the survival of these patients. The therapeutic approach of combining microwave ablation with cementoplasty in the treatment of bone metastases offers a promising perspective for the prevention of metastasis. Clinically, substantial evidence (14, 15) supports the therapeutic effects of microwave ablation on the prevention of bone metastasis progression.
Currently, the microwave ablation technology is being used as an alternative method to reduce surgical blood loss and cause revascularization. This technology leverages a microwave magnetic field to achieve irreversible coagulation and necrosis of the lesion tissue, thereby reducing tumor cells. The advantages of microwave ablation technology include preventing heat dissipation and lowering electrical and thermal conductivity, which in turn provides a complete, unified, and predictable ablation effect (15). Consequently, microwave ablation has been widely used to treat liver cancer, lung cancer, and uterine fibroids. However, patients with malignant tumors who have bone metastases often experience high pressure, necessitating high-pressure injections of bone cement. This not only frequently results in cement leakage but also promotes the transfer of tumor cells through the bloodstream, increasing the risk of cancer cell spread. Therefore, there is an urgent need to find a safe and effective treatment method for managing bone metastases. Indeed, the combination of microwave ablation with cementoplasty appears to meet the necessary requirements for treating patients with bone metastasis. This method provides pain relief, improves the patient’s physical condition, and reduces complications.
In this study, we aimed to investigate the therapeutic role of microwave ablation combined with cementoplasty for patients with bone metastasis. Previous studies have accurately assessed the treatment outcomes of microwave ablation treatment for patients with malignant tumors, which could effectively relieve the clinical symptoms and complications of malignant tumors. Cementoplasty is commonly utilized in the treatment of bone metastases arising from malignant tumors. In this study, we hypothesized that the combination of microwave ablation with cementoplasty can effectively alleviate the symptoms of patients with bone metastasis.
2. Objectives
The aim of this study was to assess the effectiveness of computed tomography (CT)-guided microwave ablation combined with cementoplasty in treating patients with bone metastasis.
3. Patients and Methods
This clinical study included a total of 20 patients with bone metastasis who were referred to the oncology ward of our hospital from January 2017 to January 2020. The study protocol was prospectively reviewed and approved by our hospital’s ethics committee. All patients provided their written informed consent to participate in the study.
3.1. Patient Enrollment
The inclusion criteria for this study were as follows:
(1) All patients included in this clinical study had a confirmed tumor history, a pathological diagnosis, and painful symptoms. The CT or magnetic resonance imaging results indicated that the location of bone metastasis was consistent with the site of percussion pain and tenderness.
(2) Patients who had metastases in other distant locations, such as the liver and lung, were in a relatively stable condition and exhibited no obvious clinical symptoms.
(3) The patients had no contraindications related to liver function, kidney function, or blood coagulation indices.
(4) All patients were treated using CT-guided microwave ablation combined with cementoplasty.
On the other hand, the exclusion criteria for this study were as follows:
(1) Patients with bone metastasis who were unwilling to undergo CT-guided microwave ablation combined with cementoplasty.
(2) Patients with an obvious tendency to bleed or active bleeding.
(3) Patients with a history of hypertension that could not be controlled after treatment.
(4) Patients who had undergone major surgery within the past 30 days.
3.2. Procedures
After obtaining consent from both the patients and their family members, the entire procedure of CT-guided microwave ablation combined with cementoplasty was carried out. Before surgery, patients were thoroughly informed about the purpose of the treatment, the expected outcomes, the main risks, potential complications, and corresponding symptoms, as well as alternative therapies. The hospital required the patients to abstain from eating and drinking for four hours prior to the surgery. First, the patients were administered local anesthesia to alleviate the pain while strictly adhering to aseptic operation techniques. The power of the microwave ablation was set at 30 - 50 W, and the ablation time ranged from 1.5 to 15 minutes. During the ablation process, the position, angle, and depth of the ablation needle were adjusted to cover the location of bone metastases. Clinicians monitored the patients’ vital signs and pain levels throughout the procedure, adjusting the ablation power and duration as needed based on the patient’s tolerance. Once the ablation was completed, the ablation needle was removed. Subsequently, the bone cement was prepared, and when it reached the drawing stage, it was slowly poured into the site of the bone metastasis that was treated via ablation under low pressure. The volume of the bone cement typically used ranged from 1.5 to 9 mL.
3.3. Safety Evaluation
Immediately after the operation, a CT scan was performed to observe the position of the bone cement. The CT scan confirmed that the cement was in an ideal position with no overflow or leakage into the surrounding tissues, blood vessels, or spinal canal, and no intraoperative complications were observed. Any major complications that occurred during the image-guided ablation or post-treatment were assessed according to the standards set by the 2005 International Working Group on Image-Guided Tumor Ablation. Major complications were considered as those that cause significant damage and dysfunction, necessitate hospitalization, or prolong the hospital stay. On the other hand, symptoms in patients that were self-limiting left no sequelae required only a short period of hospitalization or necessitated minor treatment were considered as minor complications. The general clinical data of the patients were collected during their hospitalization period.
3.4. Treatment Efficacy Evaluation
The Quality of Life Questionnaire-Bone Metastases Module 22 (QLQ-BM22) by the European Organization for Research and Treatment of Cancer was used to accurately assess the treatment efficacy for the patients. The QLQ-BM22 evaluation criteria are outlined in Table 1. A high score indicated the severity of the patient’s pain, while a lower score suggested a better quality of life. Follow-ups were conducted at one week, one month, three months, and six months post-treatment. The QLQ-BM22 scores were collected at all the mentioned time points.
| Variables | Case/Range | Proportion, % | Mean ± SD |
|---|---|---|---|
| Gender | — | ||
| Male | 14 | 70 | |
| Female | 6 | 30 | |
| Age, y | 61.3 ± 11 | ||
| <60 | 3 | 15 | |
| ≥60 | 17 | 85 | |
| Number of bone metastases | — | ||
| <3 | 12 | 60 | |
| ≥3 | 8 | 40 | |
| Metastasis position | |||
| Liver metastasis | 8 | 40 | |
| Lung metastasis | 12 | 60 | |
| Other metastases | 7 | 35 | |
| Maximum diameter of metastasis, cm | 3.25 ± 1.31 | ||
| ≤3 | 7 | 35 | |
| >3 | 13 | 65 | |
| Primary site | — | ||
| Respiratory system | 7 | 35 | |
| Digestive system | 7 | 35 | |
| Urinary tract system | 3 | 15 | |
| Others | 3 | 15 | |
| Specific primary tumors | 13 | 65 | |
| Adenocarcinoma | 3 | 15 | |
| Neuroendocrine carcinoma | 2 | 10 | |
| Squamous cell carcinoma | 2 | 10 | |
| Procedure data | — | ||
| One procedure | 3 | 15 | |
| Two procedures | 17 | 85 | |
| Surgical site | — | ||
| Thoracic spine | 12 | 40 | |
| Lumbar spine | 10 | 33.3 | |
| Pelvis | 5 | 16.7 | |
| Others | 3 | 10 | |
| Death | |||
| Six months after treatment | 4 | 20 | |
| Ten months after treatment | 7 | 35 | |
| Method of follow-up | |||
| Three-month follow-up | |||
| Six-month follow-up | Questionnaire | ||
| Survival time | 0.36 - 8 months | ||
| Median survival time | 3.23 ± 3.43 months | ||
| Ablation power, w | 30~50 | 35.46 ± 4.89 | |
| Ablation time, min | 1.3~16 | 3.68 ± 2.64 | |
| Bone cement dosage, mL | 1.8~9 | 4.39 ± 2.01 |
3.5. Survival Analysis
The quality of life was evaluated using the EuroQOL Five Dimensions Questionnaire (EQ-5D) for patients with bone metastasis. The EQ-5D score, which is calculated using the Japanese Time Trade-off (TTO) method, is widely used to assess the health status of both patients and healthy individuals, particularly for evaluating the decline in health status caused by a specific disease. A health survival status is marked as 1, while a death status is marked as 0. Any impaired health status resulting from treatment and disease progression factors was recorded within the range of 0 - 1.
Additionally, the Quality-Adjusted Life Year (QALY) measure was utilized to assess the survival status of patients with bone metastases. The QALYs were calculated by multiplying the time span of the EQ-5D and the QLQ-BM22 scores. The post-treatment time points were also in January, March, and June, respectively. Ultimately, all QALY data for patients, both pre-treatment and post-treatment, were collected over a period of six months. Patients were categorized based on their health status. A patient who was healthy was denoted as one, while a patient who expired was represented as zero. Patients with disabilities were assigned a score of 0-1 in accordance with relevant standards.
3.6. Statistical Analysis
Statistical analysis was performed using SPSS version 19.0 (IBM Corp. Released 2010. IBM SPSS Statistics for Windows, Version 19.0. Armonk, NY: IBM Corp.). All data are presented as mean ± standard deviation (SD). The choice of statistical test depended on the distribution of the data. For normally distributed data, changes between pre-treatment and post-treatment were detected using repeated measures ANOVA. For data that were not normally distributed, the Friedman test was used to observe changes between pre-treatment and post-treatment. A P-value of less than 0.05 was considered statistically significant.
4. Results
4.1. Intraoperative Complications
As shown in Table 1, the technical success rate for 30 surgical sites across 20 patients was 100%. The primary complication encountered in patients with malignant tumors and bone metastases was cement leakage. This included three cases of peripheral leakage, one case of tail loss, two cases where the cement penetrated into the blood vessels surrounding the metastasis, and one case where it penetrated into the spinal canal. According to the standards for evaluating complications, these seven cases, which represented an incidence rate of 35%, were classified as minor complications.
4.2. QLQ-BM22 Score
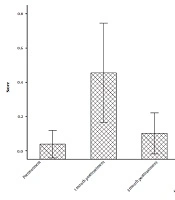
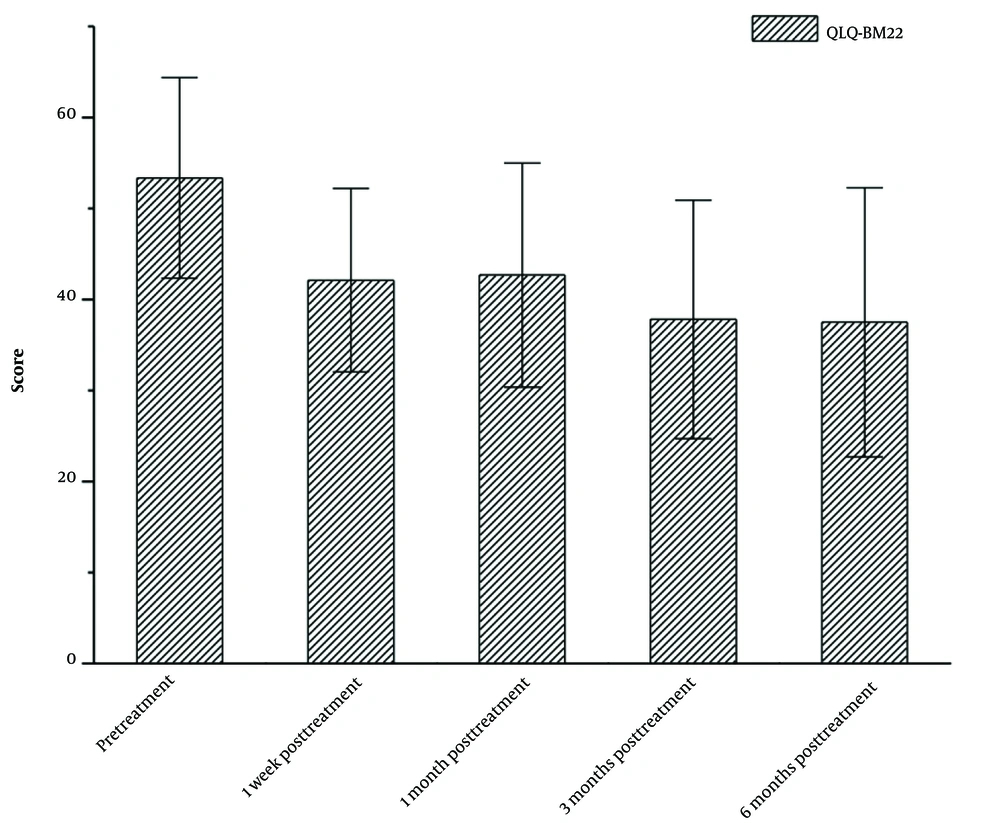
As shown in Figure 1, the median score of QLQ-BM22 showed a gradual decrease from pre-treatment to six months post-treatment. The median scores of QLQ-BM22 at pre-treatment, one week post-treatment, one month post-treatment, three months post-treatment, and six months post-treatment were measured to be 52.36, 42.12, 42.68, 37.81, and 37.50, respectively. The P-value from the Friedman test, comparing pre-treatment and six months post-treatment, was less than 0.05.
4.3. EQ-5D Score
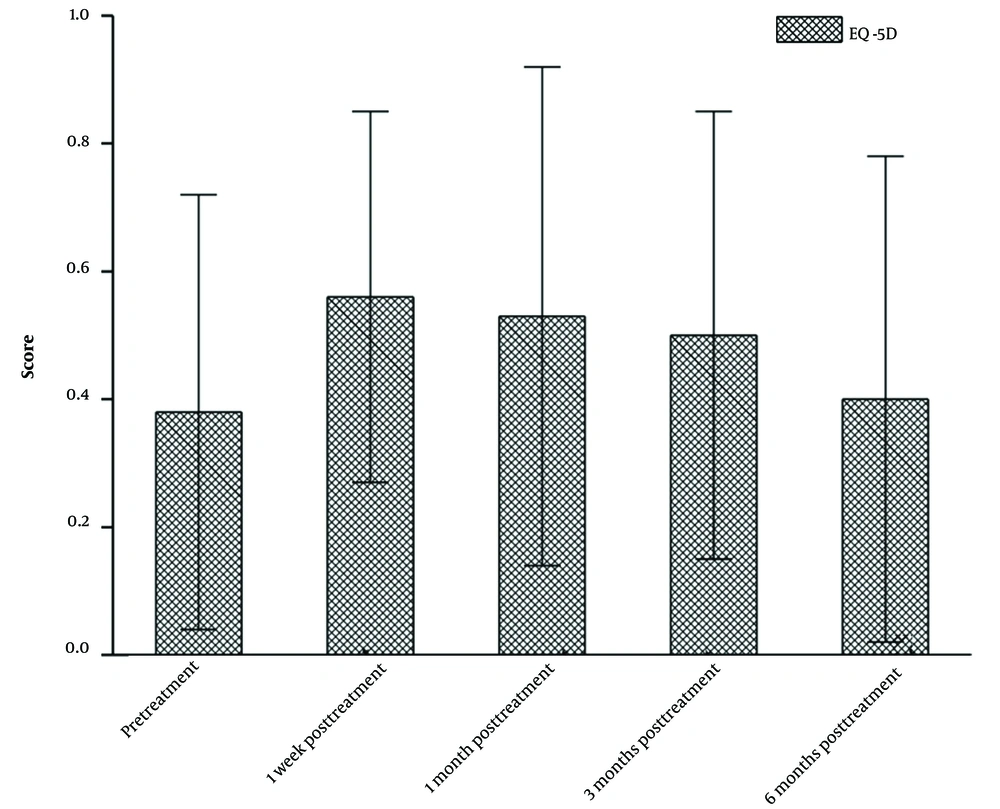
This study examined the median EQ-5D score from pre-treatment to six months post-treatment in patients undergoing microwave ablation combined with cementoplasty. As depicted in Figure 2, there was an increase in the median EQ-5D score from pre-treatment to one week post-treatment. However, the EQ-5D score gradually decreased from one week post-treatment to six months post-treatment. The median EQ-5D scores at different stages of treatment were as follows: Pre-treatment, 0.38; one week post-treatment, 0.56; one month post-treatment, 0.53; three months post-treatment, 0.50; and six months post-treatment, 0.44. Compared to the median EQ-5D score at one week post-treatment, an increasing trend was observed. Among the five evaluated aspects (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression), significant improvements were observed in the patients’ pain/discomfort and anxiety/depression, followed by mobility. Improvements were also observed in both self-care and daily activity abilities. However, the difference between the median EQ-5D scores pre-treatment and six months post-treatment was not statistically significant (P > 0.05).
4.4. Subsistence Analysis
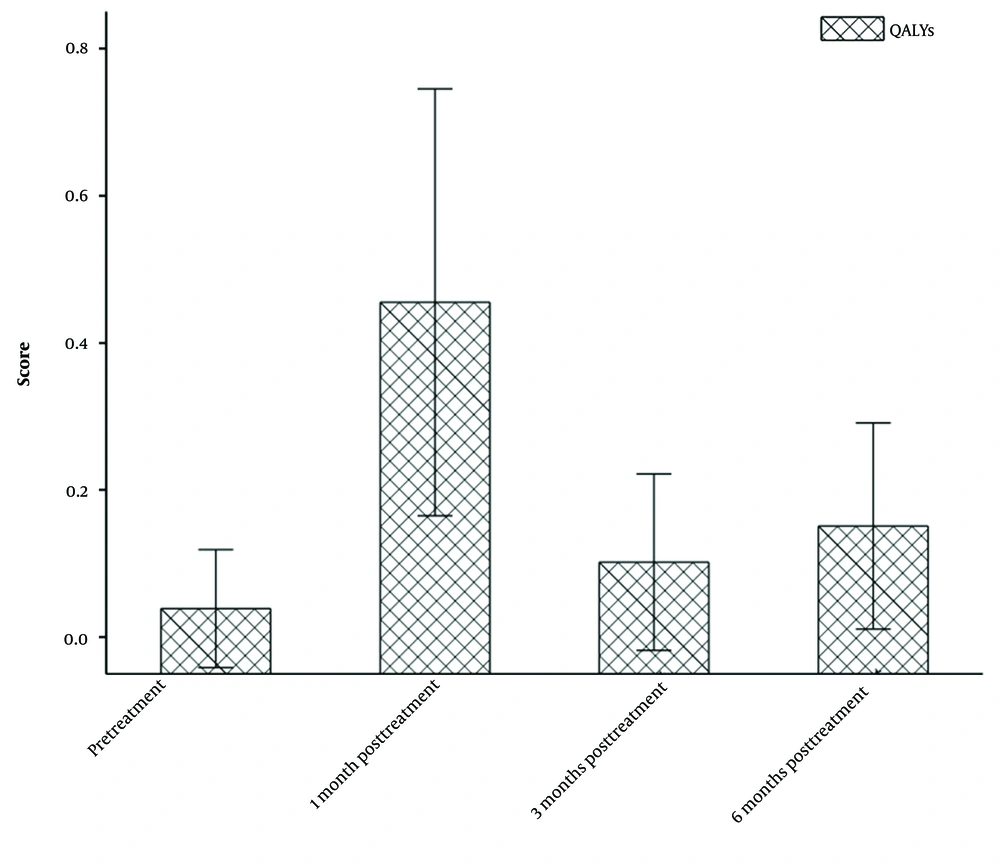
The follow-up period for the patients ranged from 1 to 20 months. Within this period, four patients expired within the first six months, and an additional three patients succumbed to the disease within 10 months. Of these seven patients, five succumbed to the progression of their primary disease, one to a severe lung infection, and one to septic shock. The survival time for these patients ranged from 0.36 to 8 months, with a median survival time of 3.23 ± 3.43 months. The QALY measure was used to evaluate the patients’ survival. The pre-treatment QALY score (n = 20) was 0.039, while the post-treatment QALY scores at 1 month (n = 20), three months (n = 20), and six months (n = 16) were 0.455, 0.102, and 0.151, respectively (Figure 3). Compared to the pre-treatment QALY score, the scores significantly increased at one month, three months, and six months post-treatment (P < 0.05), and the difference was statistically significant (Figure 3).
5. Discussion
Most patients with malignant tumors and bone metastasis commonly have a poor prognosis, a poor physical functional status, and a shorter survival. Malignant tumors pose a significant risk to the health and life of patients. Consequently, the primary objectives of treating patients with bone metastasis are to extend their lifespan and alleviate their pain (16-18). Computed tomography-guided microwave ablation combined with cementoplasty has been effectively utilized for many years in the treatment of patients with malignant tumors and bone metastasis. This approach is favored due to its short operation time, cost-effectiveness, and compatibility with other therapies (19). The bone cement is injected to stabilize and strengthen the bone of patients with malignant tumors. The process of bone cement polymerization involves a rise in temperature or the potential toxicity of the bone cement itself, both of which can effectively eliminate nociceptors and consequently alleviate pain. Simultaneously, microwave ablation, which generates heat swiftly through the high-frequency oscillation of water molecules, can proficiently manage larger-diameter lesions, thereby minimizing patient discomfort.
The conventional use of bone cement in treating patients with bone metastasis arising from malignant tumors can result in numerous intraoperative complications, including discomfort, cement leakage, and post-ablation syndrome (20, 21). In our study, factors such as age, the type of primary tumor, and the presence of other metastases were considered when evaluating the treatment efficacy in patients. Our findings suggest that age does not have a significant correlation with bone metastasis. However, the type of primary tumor and the presence of other metastases were found to be associated with bone metastasis. Notably, the application of bone cement in conjunction with microwave ablation significantly reduced the incidence of intraoperative complications.
In this regard, a previous report (22) showed that the traditional bone cement operation led to post-ablation syndrome in nearly one-third of the patients. The patients in our study were administered adequate local anesthesia, intravenous analgesia, and sedation before surgery, and the pain caused by the operation could be tolerated without post-ablation syndrome. In our study, cement leakage was observed in two patients, accounting for 10% of the cases. This is a significant reduction compared to the 20% incidence rate reported in patients undergoing traditional bone cement operations. We can conclude that CT-guided microwave ablation combined with cementoplasty can reduce the incidence of intraoperative complications in patients with bone metastasis arising from malignant tumors.
The QLQ-BM22 and EQ-5D were used to evaluate the patients’ quality of life, which showed a close relationship with the health status of patients with bone metastasis due to malignant tumors (23, 24). The QLQ-BM22, a comprehensive measure that includes factors such as the site of pain, characteristics of pain, dysfunction, and psychological impact, effectively captures the influence of pain on daily activities and the social and psychological challenges faced by patients with bone metastasis. The results of the present study indicated that the median scores of QLQ-BM22 in the pre-treatment and one week, one month, three months, and six months post-treatment were 52.36, 42.12, 42.68, 37.81, and 37.50, respectively. The P-value of less than 0.05 signifies that these results were statistically significant. This finding suggests that CT-guided microwave ablation combined with cementoplasty can reduce the pain and psychological burden of patients, prolong the survival time, and improve their quality of life.
In the current study, the EQ-5D was also used to evaluate the patients’ quality of life. The median EQ-5D scores were 0.38, 0.56, 0.53, 0.50, and 0.44 in the pre-treatment and one week, one month, three months, and six months post-treatment, respectively. However, the P-value (> 0.05) represents that the results were not statistically significant. Therefore, CT-guided microwave ablation combined with cementoplasty not only reduces the patients’ pain but also decreases their anxiety and depression. Additionally, the QALYs include the length and quality of life; therefore, this measure was utilized in this study to evaluate the survival of patients with bone metastasis. A previous study in 2009 (25) used the QALYs to investigate the patients’ duration of life and showed that the median survival of patients with lung cancer and bone metastases was 90 days.
With the development of technologies and the clinical application of comprehensive treatment methods, the median survival of patients with lung cancer and bone metastasis has been also prolonged. A study in 2018 (26) reported that the median survival time of patients with lung cancer and bone metastasis has extended to 148 days. In the present study, the median QALY score was 0.039 prior to treatment. Following treatment, the median QALY scores at one, three, and six months post-treatment were 0.455, 0.102, and 0.151, respectively. The P-value of less than 0.05, when compared to the pre-treatment median QALY score, indicates that the difference was statistically significant. It can be inferred that the combination of CT-guided microwave ablation with cementoplasty significantly extends the survival of patients with bone metastasis.
However, this study has some limitations. The sample size of our study was smaller than that of other relevant clinical studies. Therefore, we can further investigate the role of CT-guided microwave ablation combined with cementoplasty in patients with bone metastases in a larger sample size. In conclusion, this study revealed that CT-guided microwave ablation combined with cementoplasty can decrease major intraoperative complications in malignant tumors with bone metastasis, reduce the QLQ-BM22 score, and increase the EQ-5D and QALY scores. Overall, CT-guided microwave ablation combined with cementoplasty had protective effects on patients with bone metastases.