1. Context
Hepatocellular carcinoma (HCC) is a highly vascular tumor and one of the most common cancer types around the world (1). It can rapidly develop and directly invade the surrounding parenchyma and capsule, resulting in spontaneous rupture. The incidence of spontaneous HCC rupture is reportedly less than 3% in the Western population, while the highest rate is 26% in Asian countries (2-4). Generally, it is a serious and life-threatening complication, which ranks the third among the causes of HCC deaths (4-6). Although the incidence of spontaneous HCC rupture has decreased with the improvement of early diagnosis in recent years, the 30-day mortality rate is still as high as 17 - 71% (2, 3, 7-10).
Patients with spontaneous HCC rupture usually experience shock, hypoperfusion, and multiple organ dysfunction. The primary treatment goal for these patients is to achieve a stable hemodynamic state and save their lives. In previous studies, the safety and efficacy of transarterial embolization/chemoembolization (TAE/TACE) have been fully demonstrated for critically ill patients with spontaneous HCC rupture (4, 8, 10, 11). Nevertheless, the clinical outcomes of emergency TAE/TACE in patients with spontaneous HCC rupture remain unpredictable. Despite successful embolization for bleeding termination, a significant number of patients still have a short survival. In previous studies, the 30-day mortality rate of patients with spontaneous HCC rupture significantly differs following emergency TAE/TACE (7.7%~75%), and the influential factors for mortality are inconsistent (1, 4, 6, 9, 12-31). Therefore, it is necessary to conduct a meta-analysis of 30-day mortality data after emergency TAE/TACE for patients with spontaneous HCC rupture.
2. Objectives
This study aimed to conduct a comprehensive quantitative evaluation of early mortality after emergency TAE/TACE for spontaneous HCC rupture and to perform an overall analysis of risk factors to obtain more representative data.
3. Methods
3.1. Search Strategy
This study was conducted according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines (32). The PubMed/Medline, Web of Science, and Embase databases were searched for relevant publications. The used keywords included “hepatocellular carcinoma” OR “hepatoma” OR “liver cell carcinomas” OR “liver cancer” OR “hepatic carcinoma” OR “HCC” AND “rupture”. Taking PubMed as an example, the detailed search strategy is shown in Appendix 1. All databases were searched from November 1, 2000 until November 1, 2021.
3.2. Study Selection
The inclusion criteria were as follows: (1) evaluation of adult patients with a definite diagnosis of HCC and spontaneous rupture; (2) use of interventions, including TAE/TACE for emergency hemostasis; (3) evaluation of the main outcome of this study, i.e., the 30-day mortality after TAE/TACE (or our calculation based on the available data); (4) study types, including published cross-sectional studies, single-arm clinical trials, case-control studies, and randomized controlled trials; and (5) English-language publications. In the included studies, the data of single-arm clinical trials consisted of baseline data, while cohort studies and randomized controlled trials only included the TAE/TACE group data.
On the other hand, the exclusion criteria were as follows: (1) age younger than 18 years; (2) evaluation of other types of liver malignancies; and (3) poor-quality studies.
3.3. Data Extraction
According to the abovementioned inclusion and exclusion criteria, two authors (C.M. and Y.W.) selected the papers independently. Data, including the title, authors, year, and country of the study, demographic characteristics of the patients, 30-day mortality after TAE/TACE, patients’ history, liver function grade, and preoperative laboratory indicators, were extracted from the articles.
3.4. Quality Assessment
The literature quality assessment was conducted by two authors using the Downs and Black Checklist (33) (see Appendix 2).
3.5. Statistical Analysis
After the mortality data were transformed by double arcsine transformation (34), the random-effects model was used to combine the transformed effect sizes. Next, the combined mortality data and 95% confidence intervals (CI) were obtained after the formula was returned. Heterogeneity between studies was analyzed by I2 statistic, where values above 50% indicated moderate heterogeneity. If I2 values were above 50%, the source of heterogeneity was explored, and a subgroup analysis was performed according to factors that may lead to heterogeneity. Differences within subgroups were examined using the Q-value method. Additionally, a linear regression analysis was performed to evaluate the effects of confounding factors on the 30-day mortality and to find the source of heterogeneity.
In this study, a sensitivity analysis was conducted. If outlier studies were found, they were removed, and then, a combined analysis was performed after removing all sensitive items to appraise the stability of the results. The Egger’s test, Begg’s test, and funnel plots were used to evaluate publication bias. If necessary, the trim-and-fill method was employed to correct for bias (35). All the mentioned calculations and analyses were performed in Stata Version 15.1 and R Project 4.1.2. The level of statistical significance was set at P < 0.05 in all tests (two-tailed).
4. Results
4.1. Study Characteristics
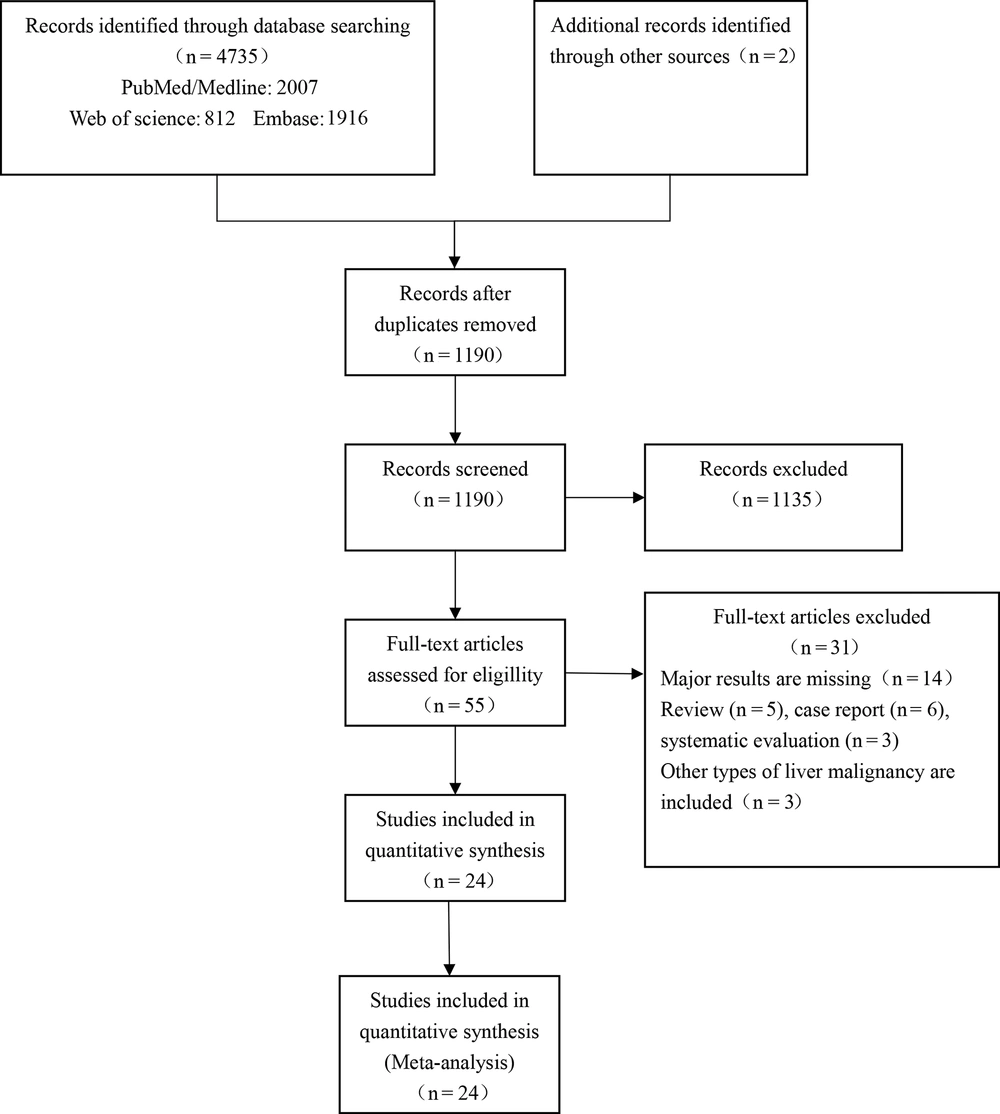
A total of 24 studies were included in this meta-analysis (Figure 1). In these studies, there were considerable variations in the patients’ age (47.4 - 69.8 years), tumor characteristics, liver functional reserve, and preoperative assay results. Of 24 studies, two (8.3%) were conducted in Europe (6, 15), one (4.2%) in North America (9), and 21 (87.5%) in Asia (1, 4, 6, 12-14, 16-31). All studies were retrospective. A summary of the characteristics of the included studies is presented in Tables 1-4. The Downs and Black checklist was used to assess the quality of the included papers (Appendix 2 and Appendix 3). Based on the evaluations, the scores of the included studies were mainly 7 - 12, and the quality of the included studies was generally average.
| Study | Year | Region | Study type | n | Male | Mean/median age (y) | Etiology | Liver cirrhosis | ||
|---|---|---|---|---|---|---|---|---|---|---|
| HBV | HCV | Non-B & non-C | ||||||||
| Cheng et al. (12) | 2021 | Taiwan | Retrospective single-arm study | 186 | 152 (81.7) | 62.0 | 83 (44.6) | 53 (25.8) | 45 (24.2) | 135 (72.6) |
| Zhou et al. (13) | 2020 | China | Retrospective cohort study | 59 | 56 (94.9) | 58.3 | 48 (81.4) | NR | NR | 38 (64.4) |
| Zou et al. (14) | 2019 | China | Retrospective cohort study | 39 | NR | NR | 35 (89.7) | 0 (0.0) | 4 (10.3) | NR |
| Patidar et al. (15) | 2019 | India | Retrospective single-arm study | 16 | 12 (75.0) | 59.0 | NR | NR | 5 (31.3) | NR |
| Lee et al. (16) | 2019 | Hong Kong | Retrospective single-arm study | 98 | 75 (76.5) | 65.0 | 59 (60.2) | NR | NR | NR |
| Zhang et al. (17) | 2018 | China | Retrospective cohort study | 53 | 49 (92.5) | 47.4 | 51 (96.2) | 3 (5.7) | 0 (0.0) | 49 (92.5) |
| Shinmura et al. (18) | 2018 | Japan | Retrospective cohort study | 51 | 41 (80.4) | 63.8 | 21 (41.2) | 14 (27.5) | 5 (9.8) | NR |
| Fan et al. (19) | 2017 | China | Retrospective cohort study | 34 | 29 (85.3) | 49.9 | 34 (100) | NR | NR | NR |
| Wu et al. (20) | 2016 | China | Retrospective single-arm study | 13 | 13 (100) | 58.1 | 12 (92.3) | 1 (7.7) | NR | 13 (100) |
| Zhong et al. (1) | 2016 | China | Retrospective cohort study | 21 | 15 (71.4) | 61.5 | 18 (85.7) | 3 (14.3) | 0 (0.0) | 21 (100) |
| Monroe et al. (9) | 2015 | USA | Retrospective single-arm study | 23 | 19 (82.6) | 59.0 | 11 (47.8) | 12 (52.2) | NR | 23 (100) |
| Yang et al. (21) | 2014 | China | Retrospective cohort study | 41 | NR | NR | NR | NR | NR | NR |
| Lin et al. (22) | 2014 | China | Retrospective single-arm study | 16 | 12 (75.0) | 60.9 | NR | NR | NR | 16 (100) |
| Jin et al. (23) | 2013 | South Korea | Retrospective cohort study | 25 | 22 (88.0) | 54.0 | NR | NR | NR | NR |
| Kim et al. (24) | 2012 | South Korea | Retrospective single-arm study | 24 | NR | NR | NR | NR | NR | NR |
| Zhang et al. (25) | 2012 | China | Retrospective single-arm study | 30 | NR | NR | NR | NR | NR | NR |
| Shin et al. (26) | 2010 | South Korea | Retrospective single-arm study | 47 | 39 (83.0) | NR | 29 (61.7) | 4 (8.5) | NR | 45 (95.7) |
| Bassi et al. (6) | 2010 | Italy | Retrospective single-arm study | 4 | 2 (50.0) | 69.8 | NR | 2 (50.0) | NR | 4 (100) |
| Li et al. (27) | 2009 | Hong Kong | Retrospective single-arm study | 62 | 53 (85.5) | 63.0 | 49 (79.0) | 3 (4.8) | 10 (16.1) | NR |
| Kirikoshi et al. (28) | 2009 | Japan | Retrospective cohort study | 16 | 14 (87.5) | 67.0 | 1 (6.3) | 11 (68.8) | 3 (18.8) | 16 (100) |
| Kung et al. (29) | 2008 | Taiwan | Retrospective single-arm study | 167 | 124 (74.3) | 58.9 | 93 (55.7) | 63 (37.7) | NR | 156 (93.4) |
| Tan et al. (30) | 2006 | Singapore | Retrospective single-arm study | 9 | NR | NR | NR | NR | NR | NR |
| Castells et al. (31) | 2001 | Spain | Retrospective single-arm study | 7 | 6 (85.7) | 67.1 | 0 (0.0) | 5 (71.4) | 2 (28.6) | 7 (100) |
| Liu et al. (4) | 2001 | Hong Kong | Retrospective single-arm study | 42 | NR | NR | NR | NR | NR | NR |
A Summary of the Characteristics of the Included Studies a
| Study | Child-Pugh classification | MELD score | BCLC stage | Tumor number | Tumor extent | Tumor size (cm) | Macrovascular invasion | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | A | B | C | D | Single | Multiple | Right lobe | Left lobe | Bilobar distribution | ||||
| Cheng et al. (12) | 90 (48.4) | 70 (37.6) | 22 (11.8) | 12 | 6 | 36 | 123 | 21 | 70 (37.6) | 116 (62.4) | NR | NR | NR | 8.4 | 58 (31.2) |
| Zhou et al. (13) | 30 (50.8) | 24 (40.7) | 5 (8.5) | NR | NR | NR | NR | NR | 31 (52.5) | 28 (47.5) | 43 (72.9) | 16 (27.1) | NR | NR | NR |
| Zou et al. (14) | 11 (28.2) | 21 (53.8) | 7 (17.9) | NR | 0 | 22 | 17 | 0 | 18 (46.2) | 21 (53.8) | NR | NR | NR | NR | NR |
| Patidar et al. (15) | NR | NR | NR | 9 | NR | NR | NR | NR | 2 (12.5) | 14 (87.5) | 12 (75.0) | 4 (25.0) | 0 | 6.7 | NR |
| Lee et al. (16) | NR | NR | NR | NR | NR | NR | NR | NR | 33 (33.7) | 65 (66.3) | NR | NR | 59 (60.2) | 10.1 | NR |
| Zhang et al. (17) | 15 (28.3) | NR | NR | 12 | NR | NR | 38 | NR | 22 (41.5) | 31 (58.5) | 21 (39.6) | 7 (13.2) | 25 (47.2) | 10 | 18 (34.0) |
| Shinmura et al. (18) | 6 (11.8) | 26 (51.0) | 15 (29.4) | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | 7.6 | 12 (23.5) |
| Fan et al. (19) | 0 (0.0) | 0 (0.0) | 34 (100) | NR | 0 | 5 | 29 | 0 | 2 (5.9) | 32 (94.1) | NR | NR | NR | NR | 27 (79.4) |
| Wu et al. (20) | 9 (69.2) | 4 (30.8) | 0 (0.0) | NR | 0 | 13 | 0 | 0 | 9 (69.2) | 4 (30.8) | 11 (84.6) | 2 (15.4) | 0 | 6.2 | NR |
| Zhong et al. (1) | 3 (14.3) | 9 (42.9) | 9 (42.9) | NR | NR | NR | NR | NR | 12 (57.1) | 9 (42.9) | NR | NR | NR | 9.0 | 9 (42.9) |
| Monroe et al. (9) | 9 (39.1) | 9 (39.1) | 5 (21.7) | 13 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | 4 (17.4) |
| Yang et al. (21) | 17 (41.5) | 17 (41.5) | 7 (17.1) | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Lin et al. (22) | 0 (0.0) | 10 (62.5) | 6 (37.5) | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | 9.9 | 8 (50.0) |
| Jin et al. (23) | NR | NR | NR | NR | 0 | 4 | 9 | 12 | NR | NR | NR | NR | NR | NR | NR |
| Kim et al. (24) | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Zhang et al. (25) | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Shin et al. (26) | 9 (19.1) | 28 (59.6) | 10 (21.3) | NR | NR | NR | NR | NR | 20 (42.6) | 21 (44.7) | NR | NR | 20 (42.6) | 8.2 | 18 (38.3) |
| Bassi et al. (6) | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | 6.1 | 2 (50.0) |
| Li et al. (27) | NR | NR | NR | NR | NR | NR | NR | NR | 40 (64.5) | 22 (35.5) | NR | NR | 23 (37.1) | NR | 18 (29.0) |
| Kirikoshi et al. (28) | 5 (31.6) | 7 (43.8) | 4 (25.0) | NR | NR | NR | NR | NR | 4 (25.0) | 12 (75.0) | NR | NR | NR | NR | 8 (50.0) |
| Kung et al. (29) | 28 (16.8) | 112 (67.1) | 16 (9.6) | NR | NR | NR | NR | NR | 49 (29.3) | 118 (70.7) | 30 (18.0) | 50 (29.9) | 87 (52.1) | NR | 64 (38.3) |
| Tan et al. (30) | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Castells et al. (31) | 2 (28.6) | 2 (28.6) | 3 (42.9) | NR | NR | NR | NR | NR | 4 (57.1) | 3 (42.9) | NR | NR | NR | NR | 1 (14.3) |
| Liu et al. (4) | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
A Summary of the Characteristics of the Included Studies a
| Study | Metastasis No. (%) | Shock No. (%) | Pre-TAE laboratory data | Procedure method | Embolization agent | Re-rupture of HCC within one month | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hemoglobin (g/L) | Creatinine (mg/dL) | ALT (U/L) | Total bilirubin (umol/L) | Albumin (g/L) | INR | AFP (ng/mL) | ||||||
| Cheng et al. (12) | 28 (15.1) | 57 (30.6) | NR | 1.15 | 42.0 | 17.1 | 31.5 | 1.2 | 122.0 | TAE | Gelatin sponge | 1 |
| Zhou et al. (13) | NR | NR | 107.9 | NR | 70.7 | NR | NR | NR | NR | TAE | PVA or gelatin sponge | 2 |
| Zou et al. (14) | 8 (20.5) | 5 (12.8) | NR | NR | NR | NR | NR | NR | NR | TACE | Gelatin sponge | 2 |
| Patidar et al. (15) | NR | 4 (25.0) | 102.8 | 0.86 | NR | 18.0 | 31.3 | 1.2 | 26947.2 | TACE | PVA or gelatin sponge | NR |
| Lee et al. (16) | NR | NR | 89.0 | 1.10 | NR | 22.2 | 29.0 | 1.3 | 208.0 | TAE | NR | NR |
| Zhang et al. (17) | 2 (3.8) | 8 (15.1) | 93.4 | NR | 54.0 | 22.2 | 31.0 | NR | 1185.0 | TACE | PVA or gelatin sponge | 0 |
| Shinmura et al. (18) | 2 (3.9) | 12 (23.5) | 89.2 | 1.50 | 121.4 | 25.1 | 28.7 | NR | 58132.0 | TAE | Gelatin sponge | NR |
| Fan et al. (19) | 8 (23.5) | 25 (73.5) | 72.4 | NR | 176.2 | 44.5 | 25.6 | NR | NR | TAE | Gelatin sponge and stainless steel coils | NR |
| Wu et al. (20) | NR | NR | NR | NR | 48.3 | 24.5 | 39.7 | NR | NR | TACE | Gelatin sponge | NR |
| Zhong et al. (1) | NR | NR | 95.7 | 1.30 | 143.2 | 24.4 | 32.6 | 1.4 | 9136.7 | TAE | Gelatin sponge | 2 |
| Monroe et al. (9) | NR | 11 (47.8) | NR | NR | 42.0 | 22.3 | 28.0 | 1.3 | NR | TAE | Gelatin sponge or coils or spherical particles or PVA | 0 |
| Yang et al. (21) | NR | NR | NR | NR | NR | NR | NR | NR | NR | TAE | NR | NR |
| Lin et al. (22) | NR | 11 (68.8) | NR | NR | 203.7 | 45.4 | 35.0 | NR | 11068.1 | TAE | NR | 6 |
| Jin et al. (23) | NR | NR | 76.0 | 1.10 | NR | 20.5 | 29.0 | 1.3 | 1345.0 | TAE or TACE | Gelatin sponge | 1 |
| Kim et al. (24) | NR | NR | NR | NR | NR | NR | NR | NR | NR | TACE | NR | 0 |
| Zhang et al. (25) | NR | NR | NR | NR | NR | NR | NR | NR | NR | TAE or TACE | NR | 2 |
| Shin et al. (26) | NR | 22 (46.8) | 78.0 | 1.30 | NR | NR | 29.0 | 1.4 | NR | TAE or TACE | PVA or gelatin sponge | NR |
| Bassi et al. (6) | NR | NR | NR | NR | NR | NR | NR | NR | NR | TAE | NR | 1 |
| Li et al. (27) | NR | 21 (33.9) | 101.8 | 1.30 | 73.3 | 39.5 | 30.2 | NR | NR | TAE | Gelatin sponge | 2 |
| Kirikoshi et al. (28) | NR | 6 (37.5) | 122.0 | 0.92 | 53.0 | 25.7 | 33.0 | NR | 9472.0 | TAE | NR | NR |
| Kung et al. (29) | NR | NR | 89.8 | 1.84 | 64.3 | 25.5 | 26.8 | 1.2 | NR | TAE | Gelatin sponge | NR |
| Tan et al. (30) | NR | NR | NR | NR | NR | NR | NR | NR | NR | TAE | NR | 1 |
| Castells et al. (31) | NR | 4 (57.1) | 85.4 | NR | NR | 35.9 | NR | NR | 109.7 | TAE or TACE | Gelatin sponge | 1 |
| Liu et al. (4) | NR | NR | NR | NR | NR | NR | NR | NR | NR | TAE | Gelatin sponge | NR |
A Summary of the Characteristics of the Included Studies
| Study | Real cause of death | Thirty-day mortality (n) | Thirty-day mortality (%) | Downs and black checklist | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Failed hemostasis | Hepatic failure | Respiratory failure | Sepsis | Gastrointestinal bleeding | Recurrent HCC rupture | Others | ||||
| Cheng et al. (12) | 16 | 12 | 3 | 3 | 2 | 1 | 1 | 38 | 20.4 | 14 |
| Zhou et al. (13) | 0 | 4 | 0 | 0 | 1 | 2 | 2 | 9 | 15.3 | 13 |
| Zou et al. (14) | NR | NR | NR | NR | NR | NR | NR | 3 | 7.7 | 11 |
| Patidar et al. (15) | NR | NR | NR | NR | NR | NR | NR | 2 | 12.5 | 11 |
| Lee et al. (16) | NR | NR | NR | NR | NR | NR | NR | 41 | 41.8 | 11 |
| Zhang et al. (17) | 5 | 4 | 3 | 0 | 0 | 0 | 1 | 13 | 24.5 | 14 |
| Shinmura et al. (18) | NR | NR | NR | NR | NR | NR | NR | 19 | 37.3 | 13 |
| Fan et al. (19) | NR | NR | NR | NR | NR | NR | NR | 9 | 26.5 | 11 |
| Wu et al. (20) | NR | NR | NR | NR | NR | NR | NR | 3 | 23.1 | 10 |
| Zhong et al. (1) | NR | 4 | NR | NR | NR | 2 | NR | 7 | 33.3 | 12 |
| Monroe et al. (9) | 1 | 5 | 0 | 0 | 0 | 0 | 1 | 7 | 30.4 | 14 |
| Yang et al. (21) | NR | NR | NR | NR | NR | NR | NR | 11 | 26.8 | 10 |
| Lin et al. (22) | 0 | 0 | 0 | 0 | 0 | 3 | NR | 3 | 18.8 | 10 |
| Jin et al. (23) | NR | 6 | NR | NR | NR | NR | NR | 14 | 56.0 | 9 |
| Kim et al. (24) | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 4 | 16.7 | 8 |
| Zhang et al. (25) | 0 | 11 | 0 | 0 | 3 | 2 | NR | 16 | 53.5 | 10 |
| Shin et al. (26) | NR | NR | NR | NR | NR | NR | NR | 12 | 25.5 | 11 |
| Bassi et al. (6) | 1 | 1 | NR | NR | NR | 1 | NR | 3 | 75.0 | 10 |
| Li et al. (27) | 5 | NR | NR | NR | NR | 2 | NR | 24 | 38.1 | 10 |
| Kirikoshi et al. (28) | 1 | NR | NR | NR | NR | NR | NR | 2 | 12.5 | 9 |
| Kung et al. (29) | NR | NR | NR | NR | NR | NR | NR | 52 | 31.1 | 11 |
| Tan et al. (30) | 4 | 0 | 0 | 0 | 0 | 1 | NR | 5 | 55.6 | 7 |
| Castells et al. (31) | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 3 | 42.9 | 10 |
| Liu et al. (4) | NR | 10 | NR | NR | NR | NR | NR | 15 | 36.0 | 9 |
A Summary of the Characteristics of the Included Studies
4.2. Meta-analysis of 30-Day Mortality After Transarterial Embolization/Chemoembolization for Spontaneous Hepatocellular Carcinoma Rupture
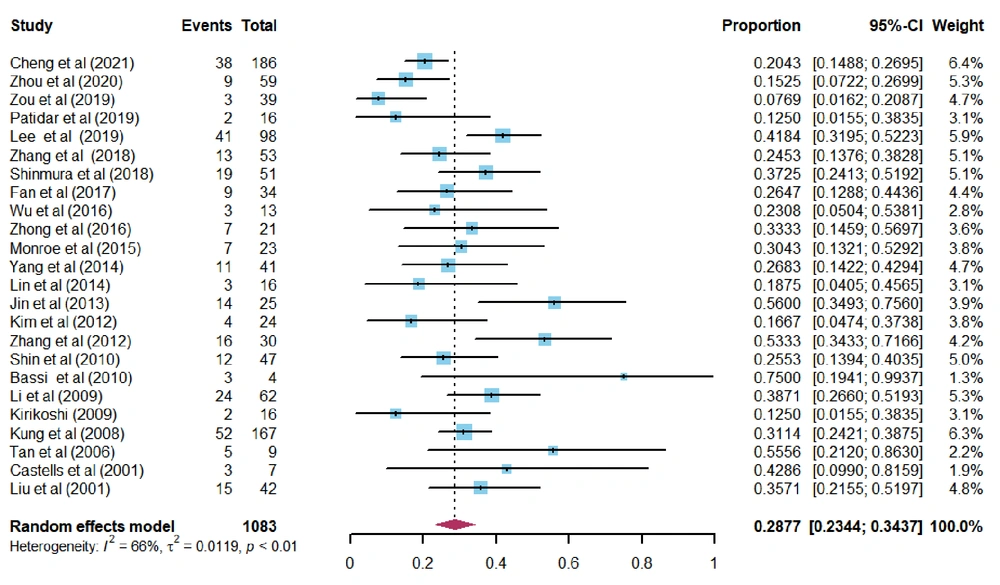
The combined 30-day mortality of patients with spontaneous HCC rupture, who underwent emergency TAE/TACE treatment (n = 1,083 in 24 studies), was 28.8% (95% CI: 23.4 - 34.4%). There was moderate heterogeneity between studies (I2 = 65.8%, P < 0.0001) (Figure 2). Hepatic failure was the most common cause of 30-day mortality after the procedure (13 studies, accounting for 42.34% of total deaths) (Tables 1-4).
4.3. Subgroup Analysis
According to the results pertaining to the influential factors in the literature, further subgroup analysis was conducted according to different factors (Table 5). The results of subgroup analysis revealed that a bilobar tumor distribution (P = 0.0011) was significantly associated with increased 30-day mortality after TAE/TACE (Figure 3A).
| Subgroups | Number of studies | Sample size (n) | Thirty-day mortality (events) | P-value of heterogeneity test | I2 value of heterogeneity test (%) | Combined mortality (95% CI) | Q-value between subgroups | P-value between subgroups |
|---|---|---|---|---|---|---|---|---|
| Region | 4.46 | 0.3469 | ||||||
| China | 9 | 306 | 74 | 0.0038 | 64.7 | 0.2418 (0.1604, 0.3329) | ||
| Korea and Japan | 5 | 163 | 51 | 0.0115 | 69.1 | 0.2646 (0.1697, 0.4361) | ||
| Other Asian countries | 7 | 580 | 177 | 0.0009 | 73.6 | 0.3180 (0.2349, 0.4069) | ||
| Europe | 2 | 11 | 6 | 0.3574 | 0.0 | 0.5468 (0.2253, 0.8521) | ||
| USA | 1 | 23 | 7 | - | - | 0.3043 (0.1304; 0.5102) | ||
| Study type | 1.19 | 0.2762 | ||||||
| Retrospective single-arm study | 15 | 744 | 228 | 0.0008 | 61.8 | 0.3113 (0.2468, 0.3793) | ||
| Retrospective cohort study | 9 | 339 | 87 | 0.0006 | 70.8 | 0.2535 (0.1684, 0.3485) | ||
| Tumor number | 0.56 | 0.4548 | ||||||
| Single tumor > 50% | 5 | 162 | 46 | 0.0432 | 59.3 | 0.2820 (0.1624, 0.4174) | ||
| Multiple tumors > 50% | 9 | 656 | 172 | 0.0005 | 71.6 | 0.2347 (0.1689, 0.3071) | ||
| Tumor extent | 10.59 | 0.0011 | ||||||
| Bilobar distribution | 4 | 374 | 129 | 0.1549 | 42.8 | 0.3451 (0.2799, 0.4132) | ||
| One lobe | 3 | 88 | 14 | 0.7240 | 0.0 | 0.1520 (0.0792, 0.2402) | ||
| Albumin (g/L) | 4.74 | 0.0934 | ||||||
| ≥ 35 | 2 | 29 | 6 | 0.7743 | 0.0 | 0.2062 (0.0703, 0.3806) | ||
| ≥ 30 - < 35 | 6 | 354 | 86 | 0.0568 | 53.4 | 0.2432 (0.1674, 0.3272) | ||
| < 30 | 7 | 445 | 154 | 0.0999 | 43.7 | 0.3473 (0.2834, 0.4139) | ||
| Hemoglobin (g/L) | 5.58 | 0.0614 | ||||||
| ≥ 120 | 1 | 16 | 2 | - | - | 0.1250 (0.0027, 0.3410) | ||
| ≥ 90 - < 120 | 5 | 211 | 55 | 0.0314 | 62.3 | 0.2481 (0.1524, 0.3570) | ||
| < 90 | 7 | 429 | 150 | 0.0962 | 44.2 | 0.3520 (0.2841, 0.4229) | ||
| Period of time | 15.80 | 0.0074 | ||||||
| (2020 - 2021) | 2 | 245 | 47 | 0.4062 | 0.0 | 0.1905 (0.1431, 0.2428) | ||
| (2018-2019) | 5 | 257 | 78 | 0.0001 | 82.5 | 0.2472 (0.1244, 0.3936) | ||
| (2016 - 2017) | 3 | 69 | 19 | 0.8068 | 0.0 | 0.2773 (0.1729, 0.3941) | ||
| (2014 - 2015) | 3 | 80 | 21 | 0.7473 | 0.0 | 0.2604 (0.1664, 0.3657) | ||
| (2012 - 2013) | 3 | 79 | 34 | 0.0052 | 81.2 | 0.4133 (0.1749, 0.6738) | ||
| (2001 - 2010) | 8 | 354 | 117 | 0.1072 | 41.2 | 0.3156 (0.2644, 0.3688) | ||
| Procedure method | 6.62 | 0.0101 | ||||||
| TAE | 15 | 829 | 245 | 0.0013 | 60.3 | 0.2957 (0.2413, 0.3529) | ||
| TACE | 5 | 145 | 25 | 0.2759 | 21.8 | 0.1613 (0.0889, 0.2477) |
Subgroup Analysis for Exploring the Source of Heterogeneity
The forest plot of 30-day mortality based on different subgroups: A, Tumor extent: Bilobar distribution and one-lobe involvement; B, Period of time: 2020 - 2021, 2018 - 2019, 2016 - 2017, 2014 - 2015, 2012 - 2013, and 2001 - 2010; C, Procedure method: Transarterial embolization/chemoembolization (TAE/TACE).
After stratification by publication time of studies, it was found that the 30-day mortality after TAE/TACE treatment for spontaneous HCC rupture has decreased significantly in the past two years (P = 0.0074); the corresponding value was 19.1% (95% CI: 14.3 - 24.3%) during 2020 - 2021 and 31.6% (95% CI: 26.4 - 36.9%) during 2001 - 2010 (Figure 3B).
Based on the comparison of TAE and TACE groups, although the P-value was 0.01 between the subgroups, the CIs of the two data groups overlapped (Figure 3C). So their difference was not statistically significant, this finding was further analyzed in the regression analysis. In some other subgroups, the number of studies and sample size were relatively small. The I2 statistic did not change significantly after the interaction between subgroups was excluded by Q-test, suggesting that other factors were not a source of heterogeneity in the 30-day mortality after TAE/TACE.
4.4. Meta-regression Analysis
To further evaluate the influential factors of early postoperative mortality after TAE/TACE and to explore the sources of heterogeneity between studies, a univariate regression analysis was performed on various possible influential factors (Table 6). The results revealed that preoperative liver cirrhosis (P = 0.0057) and bilobar tumor distribution (P = 0.0015) were significantly associated with increased 30-day mortality after TAE/TACE. Compared to earlier years (2001 - 2010), the period of time (2020 - 2021) was significantly associated with reduced 30-day mortality after TAE/TACE (P = 0.0002). All the mentioned factors may be potential causes of heterogeneity. Meanwhile, there was no significant difference between the TAE and TACE groups (P = 0.2227).
| Variables | Number of studies | Total sample size (n) | Β (95% CI) | P-value |
|---|---|---|---|---|
| Male gender (%) | 18 | 898 | -0.5528 (-1.2411, 0.1355) | 0.1155 |
| Age (y) | 17 | 851 | 0.0043 (-0.0072, 0.0159) | 0.4604 |
| Period of time (2020 - 2021 vs. 2001 - 2010) | 10 | 599 | -0.1545 (-0.2357, -0.0733) | 0.0002 |
| TACE vs. TAE | 20 | 974 | -0.0927 (-0.2416, 0.0563) | 0.2227 |
| HBV (%) | 15 | 876 | -0.0827 (-0.3309, 0.1656) | 0.5139 |
| HCV (%) | 13 | 689 | 0.1301 (-0.2042, 0.4644) | 0.7628 |
| Preoperative liver cirrhosis (%) | 12 | 612 | 0.4426 (0.1285, 0.7556) | 0.0057 |
| Child-Pugh classification B+C (%) | 14 | 720 | 0.1877 (-0.1203, 0.4957) | 0.2324 |
| Child-Pugh classification C (%) | 14 | 720 | 0.0999 (-0.1509, 0.3507) | 0.4348 |
| MELD score | 4 | 278 | 0.0938 (-0.2432, 0.4039) | 0.5853 |
| BCLC stage C + D (%) | 5 | 297 | 0.2278 (-0.3515, 0.8072) | 0.4409 |
| Multiple tumors (%) | 14 | 818 | -0.1126 (-0.5157, 0.2906) | 0.5842 |
| Bilobar tumor distribution (%) | 7 | 462 | 0.3932 (0.1498, 0.6365) | 0.0015 |
| Tumor size (cm) | 10 | 505 | 0.0305 (-0.0051, -0.0685) | 0.7763 |
| Macrovascular invasion (%) | 13 | 687 | -0.1488 (-0.5182, 0.2206) | 0.4298 |
| Metastasis (%) | 5 | 363 | -0.8402 (-2.0529, 0.3725) | 0.1745 |
| Shock (%) | 12 | 550 | 0.1655 (-0.2165, 0.5476) | 0.3958 |
| Hemoglobin (g/L) | 13 | 656 | -0.0007 (-0.0034, 0.0019) | 0.5931 |
| Creatinine (mg/dL) | 10 | 689 | 0.1042 (-0.1936, 0.4021) | 0.4927 |
| ALT (U/L) | 12 | 701 | 0.0004 (-0.0008, 0.0016) | 0.4927 |
| Total bilirubin (umol/L) | 15 | 788 | 0.0008 (-0.0063, 0.0079) | 0.8174 |
| Albumin (g/L) | 15 | 828 | 0.0012 (-0.0060, 0.0084) | 0.1476 |
| INR | 8 | 583 | 0.5390 (-0.5981, 1.6761) | 0.3529 |
| AFP (ng/mL) | 10 | 489 | 0.0000 (-0.0000, 0.0000) | 0.9600 |
| Re-rupture of HCC within one month (%) | 15 | 558 | -0.0176 (-0.0885, 0.0534) | 0.6274 |
| Real cause of death (%) | ||||
| Failed hemostasis | 12 | 489 | -0.0053 (-0.0242, 0.0136) | 0.5844 |
| Hepatic failure | 13 | 499 | 0.0016 (-0.0215, 0.0248) | 0.8889 |
| Respiratory failure | 9 | 407 | -0.0309 (-0.1076, 0.0457) | 0.4292 |
| Sepsis | 9 | 407 | -0.0355 (-0.1321, 0.0610) | 0.4709 |
| Gastrointestinal bleeding | 9 | 407 | 0.0433 (-0.0521, 0.1407) | 0.3674 |
| Recurrent HCC rupture | 12 | 494 | 0.0177 (-0.0791, 0.1144) | 0.7200 |
| Other causes of death | 6 | 352 | -0.0847 (-0.223, 0.0546) | 0.2334 |
Regression Analysis for Various Possible Influential Factors
4.5. Sensitivity Analysis
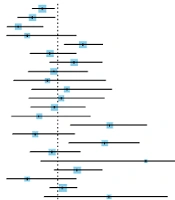
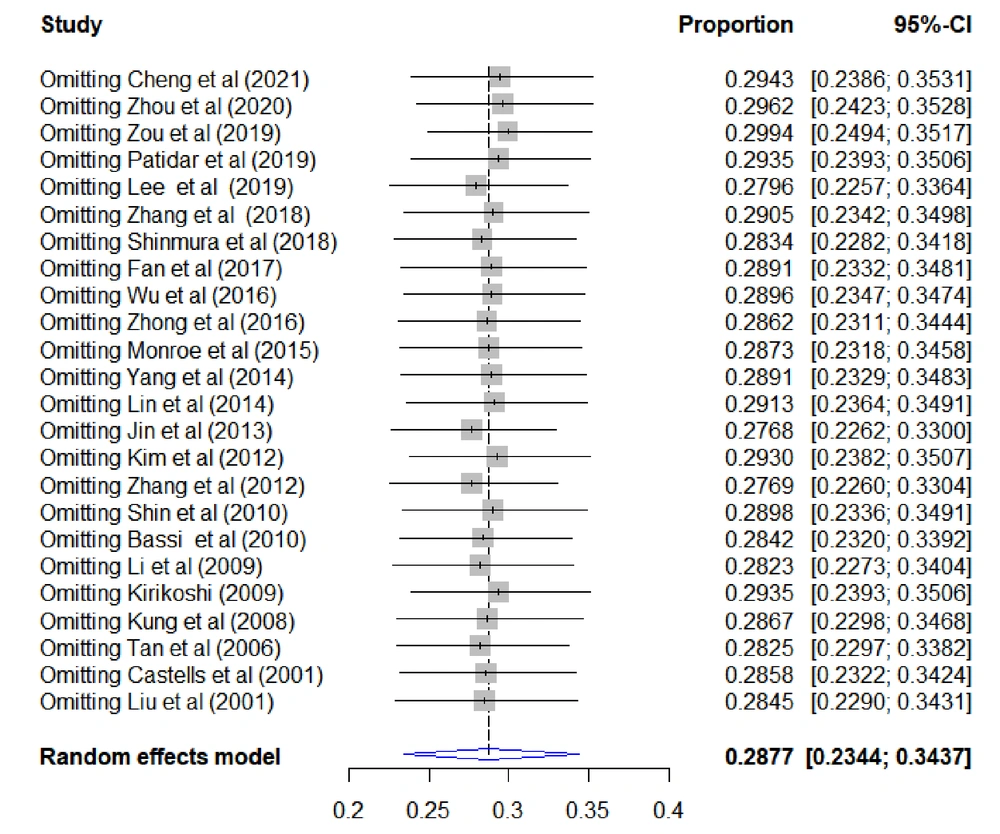
This study investigated whether sequentially excluded individual studies influenced the overall 30-day mortality after TAE/TACE for spontaneous HCC rupture (Figure 4). As shown in Figure 4, the combined effect size of the remaining studies fluctuated around 28.8%, and no significant outliers were found, indicating the acceptable stability of the included studies.
4.6. Publication Bias Analysis
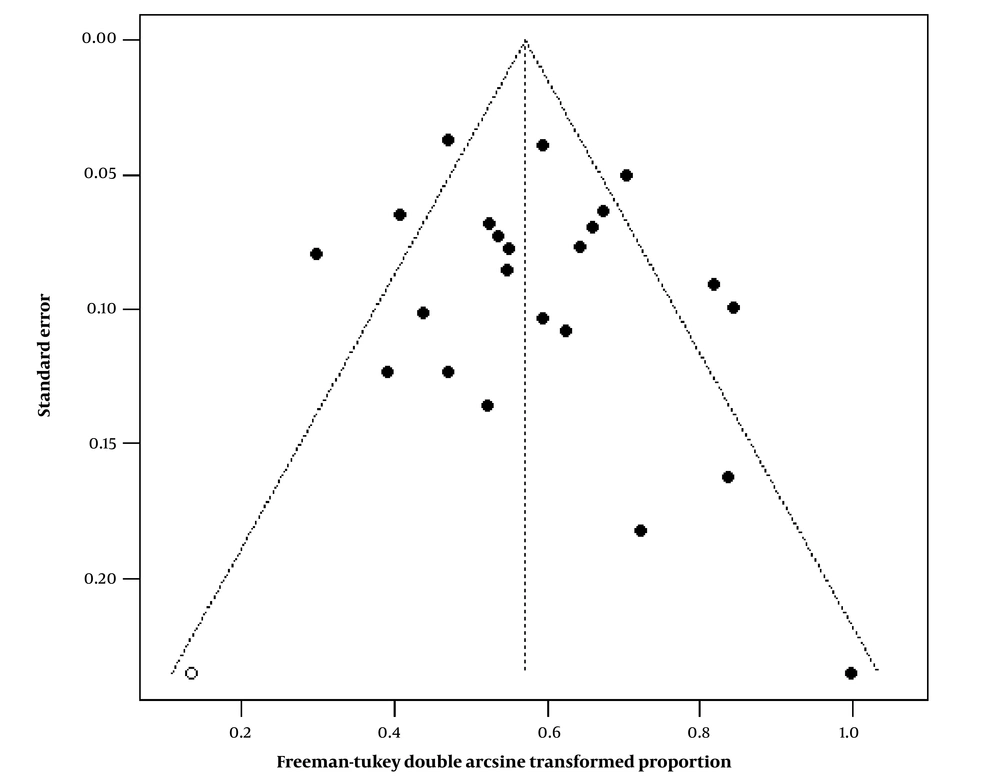
The funnel plot revealed that the merger rates of original studies were symmetrical in the upper middle part of the graph, but not in the lower half (Figure 5). The Egger’s test (P = 0.4407) and Begg’s test (P = 0.7468) were also carried out. After correction with the trim-and-fill method (Figure 5), one small sample was added to the left mirror position of the funnel plot, and the overall 30-day mortality after TAE/TACE was estimated at 28.1% (95% CI: 22.7 - 33.6%), which was not significantly different from the original result. According to this result, there was no significant publication bias in the 24 included studies.
The funnel plot of publication bias for the original studies (solid circle, the combined effect size is the double-arcsine transformed proportion of 30-day mortality). After correction with the trim-and-fill method, one study was added to the left mirror position of the funnel plot (hollow circle).
5. Discussion
Spontaneous HCC rupture is one of the most common emergency complications in advanced HCC, with a commonly poor prognosis (36). In the event of spontaneous HCC rupture, the main goal is to achieve rapid and effective hemostasis, which is the most important factor in determining early mortality (37).
Today, the main hemostatic methods for patients with spontaneous HCC rupture include conservative treatment, partial hepatectomy, and TAE/TACE. There are also some less commonly used hemostatic methods, such as perihepatic packing, suturing and folding of hemorrhagic tumors, absolute alcohol injection, and hepatic artery ligation (3). The results of conservative treatment alone are usually poor. In a multi-center study by Zhong et al. on patients with spontaneous HCC rupture, the 30-day survival was much higher after partial hepatectomy or TAE compared to conservative treatment (88.2% vs. 8.6%; P < 0.001) (1). In another study by Shinmura et al., the prognosis of TAE was better than that of conservative treatment (median survival time, 28 vs. 16 days; 30-day survival rate, 39% vs. 63%), although no significant difference was found in the overall survival rate between the two groups (18).
Currently, it is believed that conservative treatment should be only applied for dying patients with decompensation of liver function and progressive tumor, for which TAE/TACE or hepatic resection is not feasible. Hepatic resection is also one of the effective treatment options for ruptured HCC. However, relative to TAE/TACE, it is not suitable for patients with an unstable hemodynamic status or severe liver cirrhosis and coagulation dysfunction (2). Although in some previous studies, no significant difference was found in the efficacy or safety of emergency TAE/TACE and surgical resection in patients with spontaneous HCC rupture (3, 23, 37), a recent meta-analysis of the efficacy and safety of TAE/TACE and emergency surgery for spontaneous HCC rupture reported that the incidence of complications in the TAE/TACE group was only one-third of the emergency surgery group (odds ratio (OR): 0.36; P < 0.0001). The in-hospital mortality rate in this group was also half the rate reported in the emergency surgery group (OR: 0.52; P = 0.03) (38).
In previous research, the success rate of emergency TAE/TACE hemostasis in patients with spontaneous HCC rupture was as high as 53 - 100% (2, 37), and the early postoperative mortality rate was 7.7 - 75% (1, 4, 6, 9, 12-31). After combining the results of previous studies, the 30-day mortality of patients with spontaneous HCC rupture after emergency TAE/TACE was 29.0% (95% CI: 23.7 - 34.5%), which is significantly lower than the previously reported rate in these patients undergoing emergency open surgery (28 - 75%) (8).
In the present study, the 30-day mortality following TAE/TACE treatment for spontaneous HCC rupture has decreased significantly in the past two years compared to earlier years (19.1% in 2020 - 2021 vs. 31.6% in 2001 - 2010); apparently, the mortality rate is about 12% lower than earlier years, which is clinically important. This finding may be related to the following phenomena. First, development of magnetic resonance imaging (MRI), enhanced computed tomography (CT) scan, contrast-enhanced ultrasonography (CEUS), and other techniques has made the diagnosis of ruptured tumors more rapid, and it is now simpler to identify the location of ruptured tumors more accurately and achieve successful embolization. Second, with the development of interventional instruments and technologies, many previously inaccessible microvessels can now be successfully entered for more precise embolization (39, 40). Finally, with the progress of intensive care management, active initial resuscitation, effective correction of hypovolemic shock, and increased awareness of the importance of preventing decompensated liver failure in patients with potential liver cirrhosis, early mortality after TAE/TACE can be reduced.
The factors contributing to early mortality after emergency TAE/TACE in patients with spontaneous HCC rupture vary greatly in previous studies. In the current study, liver cirrhosis was an important factor affecting the early mortality of TAE/TACE in patients with spontaneous HCC rupture. HCC has always been recognized as the leading cause of death in patients with liver cirrhosis. Regardless of the stage of liver cirrhosis, 1 - 8% of patients develop HCC every year (41, 42). Zhu et al. found that liver cirrhosis is an independent predictor of spontaneous HCC rupture (43). Following cirrhosis, the liver microenvironment undergoes a series of changes. Through changes in the biomechanical properties of the liver, secretion of specific cytokines, and activation of various signaling pathways, tumor growth can be stimulated, and resistance to chemotherapy drugs can be developed (44); these characteristics may reduce the efficacy of TAE/TACE (44, 45).
In TAE/TACE, polyvinyl alcohol (PVA), embosphere, gelatin sponge particles, lipiodol, or chemotherapeutic agents emulsified with lipiodol are usually used to block the ruptured tumor blood supply artery to achieve the purpose of hemostasis and induce tumor ischemic necrosis. When liver cirrhosis occurs, the production of endothelin-1 increases, the sensitivity of its receptors enhances, and the production of nitric oxide decreases (46). After acting on hepatic stellate cells (HSC), they cause vascular remodeling in the hepatic sinusoid (contraction of HSC), which increases vascular resistance (46); consequently, embolic agents may not reach more distant and thinner blood vessels of the liver tumor, thereby reducing the effect of TAE/TACE.
Additionally, patients with liver cirrhosis often have poorer liver functional reserves, a higher risk of infection, potential coagulation disorders, and pancytopenia (due to portal hypertension and hypersplenism) (26, 27, 47). The combined effects of these factors may be also an important reason for the high early mortality rate after TAE/TACE in patients with spontaneous HCC rupture in liver cirrhosis (26, 27, 46). Tan et al. found that liver cirrhosis was an important factor, affecting the increase in 30-day mortality in patients with spontaneous HCC rupture (30). Moreover, in a multicenter study by Zhong et al., liver cirrhosis was an independent factor influencing the overall survival rate of patients with spontaneous HCC rupture (1).
In the current study, bilobar tumor distribution was an important factor affecting early mortality after TAE/TACE in patients with spontaneous HCC rupture. First, bilobar tumor distribution indicates a poor liver functional reserve (16), resulting in greater susceptibility to ischemic injury by TAE/TACE, which is closely linked to early liver failure (12). Second, bilobar tumor distribution suggests that a larger embolization area may be needed during TAE/TACE (both left and right hepatic arteries need to be entered, selective embolization should be carried out, and sometimes, the embolization scope is inevitably expanded), while the probability of post-embolization syndrome, liver failure, liver abscess/biloma, and other complications of large-scale embolization is greatly increased (9, 48). In some studies, extensive bilobar tumor involvement is even considered an absolute contraindication for TACE (48, 49). In an early study by Shin et al., bilobar tumor distribution affected the poor prognosis of patients with spontaneous HCC rupture after TAE/TACE (26). In another retrospective study, bilobar tumor distribution was an independent predictor of increased 30-day mortality after TAE in patients with spontaneous HCC rupture (OR = 29.6; P < 0.001) (16), which is consistent with the results of the present study.
In our subgroup analysis, based on the comparison of the TAE group with the TACE group, the P-value was 0.01 between the subgroups. However, the CIs of the two data groups overlapped. In the regression analysis, no significant difference was found between the TAE and TACE groups; based on the results, TACE and TAE do not appear to have different effects on the patients’ 30-day mortality. In some early studies, it was believed that TAE should be performed for hemodynamically unstable patients, while TACE is feasible for patients with a relatively stable status (4, 6, 25-31). However, in recent years, this view has not been widely accepted. Some studies suggest that for hemodynamically unstable patients with an apparent continuous hemorrhage, TACE can be considered if the liver functional reserve is not very poor (3, 17). Many centers also choose emergency TACE for patients with shock (14, 15). Nevertheless, compared to TAE, the use of chemotherapy drugs may cause further damage to the liver function. Our findings also suggest that hepatic failure is the most common cause of 30-day mortality after the procedure.
In most recent studies, TAE is still used more commonly than TACE in relatively “critically ill” patients, especially those with a poor liver function (1, 18-20, 22). Clinically, it is unclear whether TAE or TACE is superior for patients with spontaneous HCC rupture. The type of embolization agents used during TAE/TACE is important regardless of whether chemotherapeutic agents are used, but is not accurately described in most papers (Tables 1-4), and we were unable to conduct further subgroup and regression analyses. Also, there is no literature directly comparing the efficacy of these interventional strategies for patients with spontaneous HCC rupture; these questions warrant further analysis. In the current study, no detailed subgroup or regression analysis was performed. Nonetheless, the number of papers in many subgroups was limited after stratification, which can be considered a limitation. Also, this may be the reason why many other factors proposed in other papers, possibly contributing to an increase in early mortality after TAE/TACE, were not significant in our study; these factors can be also significant if the number of studies was large enough. We can simply divide the mentioned factors into three categories.
The first category includes indicators of poor liver functional reserve, including the model for end-stage liver disease (MELD) score, Child-Pugh classification, and bilirubin level (9, 12, 13, 17, 18, 24, 26, 29, 30). In many studies, the MELD score was an independent predictor of increased 30-day mortality after TAE/TACE in patients with spontaneous HCC rupture (9, 12, 17). However, the optimal critical value remains controversial. In some studies using the Child-Pugh classification, a Child-Pugh score ≥ 8 was significantly associated with poor prognosis following TAE/TACE in patients with spontaneous HCC rupture (12, 13, 18, 24, 26, 29, 30). Meanwhile, compared to conservative treatment, patients with spontaneous HCC rupture and Child-Pugh scores of 12/13 showed no significant advantage for TAE/TACE (19). Regarding the bilirubin level, although it has been included in the Child-Pugh score, there are still many studies analyzing bilirubin level as a separate influential factor. Despite the fact that the total serum bilirubin level is the main factor affecting early mortality after TAE/TACE, the optimal critical value is still unclear (12, 14, 16, 26, 27, 29).
The second category includes indicators of bleeding severity after HCC rupture, including shock on admission, hemoglobin level, albumin level, and blood transfusion volume (4, 13, 22, 24, 25, 27, 29). During hemorrhagic shock, the function of oxygen transport decreases, tissue perfusion reduces, and cell hypoxia causes serious damage to important organs. Moreover, hemorrhagic shock makes the liver function more fragile than usual, and coagulation dysfunction of patients with impaired liver function can further increase the risk of shock death. Kim et al. found that the 30-day postoperative mortality rate of TACE was 16.7% in patients with ruptured HCC, and a higher preoperative hemoglobin level was an independent influential factor reducing the postoperative mortality rate (P = 0.036) (24).
Kung et al. analyzed the prognosis of 167 patients with spontaneous HCC rupture, accompanied by hemodynamic instability after TAE and found that patients who died early had lower hemoglobin and albumin levels and more blood transfusions (P < 0.05 for all) (29). Additionally, in a retrospective study by Li et al., the early death of patients with spontaneous HCC rupture treated with TAE was associated with a low hemoglobin level, low serum albumin level, and prolonged prothrombin time (27). Serum creatinine level is also an important index reflecting the systemic hemodynamic status of critically ill patients. The significant increase in serum creatinine level usually represents greater blood loss. In the study by Kung et al., along with lower hemoglobin and albumin levels and higher transfusion volume, a serum creatinine level ≥ 1.5 mg/dL was an independent predictor of increased 30-day mortality (29).
Finally, the third category includes large tumor diameter, high alpha-fetoprotein (AFP) level, portal vein tumor thrombus formation, distant metastasis, and absence of tumor capsule, all of which suggested a significant increase of tumor load (12, 17-19, 22, 26, 28-30). In a retrospective study by Zhang et al., in addition to the MELD score, AFP ≥ 1000 ng/mL, maximum tumor diameter ≥ 10 cm, and absence of capsules around tumors were independent risk factors for 30-day mortality after TACE (17). Shinmura et al. found that distant metastasis is an independent prognostic factor for TAE and conservative treatment in patients with spontaneous HCC rupture (P = 0.023) (18). In patients without distant metastasis, the formation of portal vein tumor thrombus is an important prognostic factor (P = 0.015) (18). These important influential factors should be considered by clinicians before TAE/TACE treatment for patients with spontaneous HCC rupture.
This study had some limitations. First, there are relatively few high-quality studies on early mortality after TAE/TACE for spontaneous HCC rupture in this meta-analysis. Many studies did not include complete confounding factors; therefore, some factors proposed in many other studies, which might have contributed to an increase in early mortality after TAE/TACE, were not found significant in our study. Second, the number of studies in many subgroups was very small after stratification; consequently, the statistical efficiency of the estimated mortality in some subgroups may be insufficient. Third, the current study was not registered, and there may be small bias; nevertheless, we strictly adhered to the PRISMA guidelines. It is necessary to conduct prospective large-scale randomized clinical trials to further investigate the effect of TAE/TACE and other methods on the early mortality of spontaneous HCC rupture.
In conclusion, in recent years, the early mortality rate following emergency TAE/TACE for spontaneous HCC rupture has been significantly lower than before, but it is still not negligible. Before TAE/TACE, it is necessary for clinicians to predict the adverse outcomes, along with the patients' risk factors and disease-related factors, and to formulate appropriate intervention measures.