1. Background
Breast cancer, with a lifetime risk of 12.3%, is the most common cancer among women in the United States (1, 2). Early screening for breast cancer has led to a great decrease in the mortality rates around the world (3). There are now numerous diagnostic tools available for breast cancer screening, which include ultrasonography, 2D mammography, magnetic resonance imaging (MRI), and more recently, tomosynthesis (4-6). Conventional mammography (2D) remains the recommended standard of care according to international guidelines. While it is cost-effective and widely available in most centers, its limitations, such as low sensitivity and the potential for false-positive results, particularly in dense tissues, can sometimes necessitate additional imaging. This not only raises concerns, but also leads to additional expenses for patients (7, 8).
Tomosynthesis, also known as 3D mammographic imaging, was initially approved by the Food and Drug Administration (FDA) in February 2011. This technology generates mammographic images in 1-mm sections (9, 10). Tomosynthesis has been demonstrated to outperform conventional mammographic studies, as it produces a greater number of images with thin sections, which reduces the need for more costly diagnostic imaging procedures (11, 12). A 2020 meta-analysis by Alabousi et al. highlighted ongoing debates about the additional benefits of tomosynthesis versus conventional mammography. However, their findings suggested that tomosynthesis was more sensitive than conventional mammography in detecting breast cancer in patients with an average risk. Furthermore, they proposed that tomosynthesis alone could be sufficient for detecting breast cancer, without the need for concurrent conventional mammography (13). Meanwhile, the global adoption of this novel technique remains limited. This could be attributed to the scarcity of comprehensive data, insufficient information, or a deficit in the required expertise for its implementation. Therefore, further research is imperative to shed light on the effectiveness of tomosynthesis in detecting breast lesions.
2. Objectives
In this research, we aimed to evaluate the supplementary benefits of tomosynthesis in diagnosing breast cancer and in determining the Breast Imaging-Reporting and Data System (BI-RADS) score. This assessment was conducted on patients who had previously undergone full-field digital mammography (FFDM) and presented with an indeterminate BI-RADS score.
3. Patients and Methods
3.1. Study Design and Patient Population
This cross-sectional study was conducted from January 2019 to December 2020. The inclusion criteria were as follows: (1) All female individuals aged 30 years or older, (2) individuals who underwent both FFDM and tomosynthesis for either screening or diagnostic purposes, and (3) women who had a maximum interval of two weeks between screening and diagnosis during the study period. On the other hand, those who had undergone additional diagnostic procedures (e.g., MRI) for any reason, those who had a biopsy performed, and those who declined participation in the study were excluded.
3.2. Variables And Measurements
Demographic and clinical data of the participants were collected from medical records. This data included age, the reason for breast imaging (whether for screening or diagnostic purposes), family history of breast, uterine, or ovarian cancer, the type of mammography performed (either unilateral or bilateral), and the type of breast composition.
3.3. Ethical Considerations
All procedures in this study adhered to the guidelines of the Declaration of Helsinki. The study received approval from the Ethics Committee of Tehran University of Medical Sciences (Ethical Code: IR.TUMS.IKHC.REC.1400.308). Furthermore, consent for the use and publication of anonymized data was obtained from the patients.
3.4. Image Acquisition
All breast mammograms and digital breast tomosynthesis were conducted following standard-of-care protocols, with each breast meticulously compressed and positioned. The images were captured by a renowned center for breast radiology in Tehran, Iran. A commercially available system (AMULET Innovality, Fuji, Japan) was utilized, and with a single compression per view, digital mammography and tomosynthesis were employed to acquire two views (craniocaudal and mediolateral oblique) of each breast.
3.5. Image Interpretation
All mammography and tomosynthesis studies were scrutinized by an expert radiologist with a minimum of 12 years of experience in breast imaging. The BI-RADS scores were determined based on the assessment of micro-calcification, mass, focal symmetry, and distortion. Subsequently, the digital breast tomosynthesis (DBT) images were evaluated after one month. The 2013 guidelines from the American College of Radiology were utilized to classify breast lesions. For each patient, the DBT images were assessed for (1) the presence and appearance of micro-calcification (amorphous, coarse heterogeneous, dystrophic, group punctate, linear, pleomorphic, and scattered); (2) the presence and shape of any mass; (3) focal asymmetry; (4) distortion; and (5) BI-RADS scoring.
3.6. Statistical Analysis
SPSS version 20 (IBM Corp. Released 2011. IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp.) was used for data analysis. The mean and standard deviation were used to express quantitative data, while frequency and percentage were used to express qualitative data. The McNemar test was also used to compare the results of FFDM and DBT. The significance level was set at P<0.05.
4. Results
The study included 386 female patients, with the vast majority (97.7%) undergoing mammography and tomosynthesis as part of their regular screening. The participants’ age ranged from 31 to 97 years, with a mean age of 44.7±7.9 years. A positive family history of breast, uterine, and/or ovarian cancer was reported in 83 individuals (21.5%). In most cases, mammography was conducted bilaterally. The most frequently identified breast composition type was type C, indicating relatively dense breasts. The patients’ characteristics are summarized in Table 1.
| Variables | Value |
|---|---|
| Age, y, (range) | 44.7 ± 7.9 (31-79) |
| Breast imaging indication | |
| Screening | 377 (97.7) |
| Diagnostic | 9 (2.3) |
| Family history of breast, uterine, and/or ovarian cancer | |
| Positive | 83 (21.5) |
| Negative | 303 (78.5) |
| Breast composition | |
| A | 9 (2.3) |
| B | 29 (7.5) |
| C | 214 (55.4) |
| D | 132 (34.2) |
a Values are presented as No. (%) or mean ± SD.
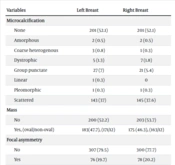
Upon reviewing the tomosynthesis images, it was observed that more than half of the cases showed no microcalcification. However, the scattered pattern was the most common, appearing in approximately 37% of each breast, while other calcification patterns were rare (Table 2).
| Variables | Left Breast | Right Breast |
|---|---|---|
| Microcalcification | ||
| None | 201 (52.1) | 201 (52.1) |
| Amorphous | 2 (0.5) | 2 (0.5) |
| Coarse heterogenous | 3 (0.8) | 1 (0.3) |
| Dystrophic | 5 (1.3) | 7 (1.8) |
| Group punctate | 27 (7) | 21 (5.4) |
| Linear | 1 (0.3) | 0 |
| Pleomorphic | 1 (0.3) | 1 (0.3) |
| Scattered | 143 (37) | 145 (37.6) |
| Mass | ||
| No | 200 (52.2) | 203 (53.7) |
| Yes, (oval/non-oval) | 183(47.7), (171/12) | 175 (46.3), (163/12) |
| Focal asymmetry | ||
| No | 307 (79.5) | 300 (77.7) |
| Yes | 76 (19.7) | 78 (20.2) |
| Distortion | ||
| No | 364 (94.3) | 356 (92.2) |
| Yes | 19 (4.9) | 22 (5.7) |
a Values are presented as No. (%).
Regarding mass lesions, there was no significant difference between the two breasts, with a mass being detected in nearly half of the radiographs for each breast, predominantly of an oval shape. Focal asymmetry and distortion were less common features, appearing in about 20% and 5% of the cases, respectively. The patients’ tomosynthesis findings are presented in Table 2.
Regarding the BI-RADS classification, 152 patients (39.4%) initially had a BI-RADS score of 0, indicating that their assessment was incomplete and indeterminate, necessitating further evaluation. After tomosynthesis, only one out of these 152 cases remained at BI-RADS 0, while the rest received a definitive BI-RADS score (P<0.001). BI-RADS 2 and BI-RADS 3 were identified in 81 (53.3%) and 45 (29.6%) of the cases, respectively, suggesting benign or likely benign results. Conversely, among these 152 patients, 25 were found to have a lesion in tomosynthesis: BI-RADS 4 in 19 patients (12.5%) and BI-RADS 5 in six patients (3.9%). It is noteworthy that the mammography scoring was completely congruent with tomosynthesis for patients with mammography BI-RADS scores of 1-6. In general, when comparing the FFDM and DBT methods, DBT clarified 99.3% (151 out of 152) of indeterminate BI-RADS 0 scores. The BI-RADS scores of FFDM and DBT are compared in Table 3.
| Variable | Tomosynthesis | Value | ||||||
|---|---|---|---|---|---|---|---|---|
| BI-RADS 0 | BI-RADS 1 | BI-RADS 2 | BI-RADS 3 | BI-RADS 4 | BI-RADS 5 | BI-RADS 6 | ||
| FFDM | ||||||||
| BI-RADS 0 | 1 (0.7) | 0 | 81 (53.3) | 45 (29.6) | 19 (12.5) | 6 (3.9) | 0 | 152 |
| BI-RADS 1 | 0 | 20 (100) | 0 | 0 | 0 | 0 | 0 | 20 |
| BI-RADS 2 | 0 | 0 | 142 (100) | 0 | 0 | 0 | 0 | 142 |
| BI-RADS 3 | 0 | 0 | 0 | 37 (100) | 0 | 0 | 0 | 37 |
| BI-RADS 4 | 0 | 0 | 0 | 0 | 27 (100) | 0 | 0 | 27 |
| BI-RADS 5 | 0 | 0 | 0 | 0 | 0 | 4 (100) | 0 | 4 |
| BI-RADS 6 | 0 | 0 | 0 | 0 | 0 | 0 | 4 (100) | 4 |
| 1 (0.3) | 20 (5.2) | 223 (57.8) | 82 (21.2) | 46 (11.9) | 10 (2.6) | 4 (1) | 386 | |
Abbreviations: FFDM, full-field digital mammography, BI-RADS, breast imaging-reporting and data system.
a Value are presented as No. (%).
5. Discussion
Traditional mammography has long been employed for the identification of breast lesions and has significantly enhanced patient survival over the past several decades. However, it falls short in terms of high sensitivity and specificity, leading many patients to require additional diagnostic procedures. This not only heightens patients’ anxieties, but also escalates the overall costs (14, 15). Therefore, there is a demand for more precise diagnostic methods for detecting breast cancer. Tomosynthesis was initially introduced in 1978 by Dr. Kopans. He discerned that low-dose X-ray images captured from various angles could be utilized to construct multiple imaging slices, thereby enhancing diagnostic accuracy and reducing X-ray absorption. However, the patent was only registered much later, in 1999, and ultimately received approval from the US FDA in 2011 (16).
Numerous recent trials have aimed to explore the role of tomosynthesis, yet further investigations are required to clarify the risks and benefits of this novel technique. In our study, we sought to determine the additional value of tomosynthesis in comparison to FFDM for the detection of breast masses. We discovered that out of 152 patients (39.4%) with a BI-RADS score of 0 in mammography, additional tomosynthesis was required for almost all of them (151 out of 152 patients), which proved to be beneficial.
Numerous recent studies have suggested that traditional 2D mammography may be becoming obsolete and may fail to detect breast masses in certain cases (17-21). Dong reported that ultrasonography enhanced the cancer detection rate by 11.9% in a large cohort of 32,000 patients who underwent both breast mammography and ultrasonography. The study concluded that ultrasonography is advisable for BI-RADS 0 to 2, particularly for individuals with dense breasts or benign breast disease, following a negative mammography result (22). Furthermore, Dang et al. suggested that the integration of tomosynthesis with mammography reduced the time required for image interpretation in comparison to using mammography alone (23). This can be attributed to the fact that the radiologist evaluates a mass or distortion from various angles and scrolls through numerous images. As a result, fewer lesions would remain undetermined or unclassified. This finding aligns with our study, as a significant number of patients required a secondary assessment using tomosynthesis.
Additionally, Bernardi et al. found that supplementing 2D mammography with 3D mammography resulted in the diagnosis of more patients with breast cancer. However, this led to an increase in false-positive recalls. They concluded that their findings should be interpreted with caution, taking into account the benefits for some patients and the potential for over-diagnosis in others. Essentially, while tomosynthesis improved the detection of breast cancers, it also led to unnecessary biopsies (24). One limitation of our study was that we did not track our patients who underwent breast biopsies. In contrast, Friedewald et al. (25) conducted a large-scale study involving nearly half a million patients divided into two groups (300,000 underwent digital mammography and 200,000 underwent digital mammography + tomosynthesis). They concluded that the addition of tomosynthesis to mammography reduced the recall rate for additional imaging and increased the detection rate of breast cancer. This finding contradicts the results of a study by Bernardi et al., which could be attributed to the varying nature of breast cancer in different geographical areas or differences in study designs. Nevertheless, there is still a need for more research in this field (24).
In another study, Rose evaluated the recall or biopsy rates, cancer detection rates, and positive predictive values in patients who had undergone tomosynthesis following mammography. They reported an increase in the cancer detection rate to 4.3 (up from 2.8) per 1000 examinations. Additionally, they noted a significant reduction in recall rates for additional imaging (26). Numerous other studies have compared the benefits of tomosynthesis following mammography, and the results have been promising. Our findings highlight the advantageous aspect of tomosynthesis in reducing the rate of BI-RADS 0. This greatly aids patients and physicians in reaching a definitive conclusion, thereby eliminating the need for further imaging and associated costs. However, tomosynthesis is not widely accessible globally.
Most studies in the literature have compared tomosynthesis plus mammography to mammography alone. In contrast, our study compared tomosynthesis alone with mammography, yielding very encouraging results. We acknowledge some limitations in our study, such as not following up with patients after imaging to evaluate the results of breast biopsies. It is recommended to conduct larger, multi-centric clinical trials with longer follow-up periods to clarify the exact supplementary role of tomosynthesis compared to mammography. This could lead to the broader acceptance of tomosynthesis. It would be also beneficial to consider training courses for radiologists and technicians to familiarize them with this new technique.
In conclusion, tomosynthesis was able to categorize almost 99.3% of patients with a BI-RADS 0 score on mammography as BI-RADS 2 to 5. This significantly aids in improving the diagnosis. Tomosynthesis enhanced the detection rate of breast masses when compared to mammography. It could clarify unclear mammography BI-RADS scores in over 99% of cases, suggesting that it could be the primary supplementary imaging modality for indeterminate BI-RADS scores.