1. Background
Pulmonary mass-like lesions are one of the common manifestations of respiratory diseases, requiring a differential diagnosis. It is critical for clinicians to confirm the diagnosis of pulmonary mass-like lesions for early and accurate therapeutic planning. However, differential diagnosis is a major difficulty in imaging studies. In the big data era, precision medicine has been the focus of clinical studies. As a new examination technology, spectral computed tomography (CT) not only provides valuable information on the morphological characteristics of lesions and their enhancement patterns to facilitate a qualitative or quantitative assessment for tumor detection, characterization, staging, and efficacy analysis, but also yields single-energy virtual images and several quantitative parameters, such as iodine concentration (IC), slope of the energy spectrum curve, and effective atomic number (Zeff), using a substance-separation technology for a more accurate analysis of the lesion components (1). The results of previous studies suggest that spectral CT scan can play a crucial role in pathological staging (2-4), while there are few studies on the application of spectral CT for the detection of mass-like lesions of the lungs.
2. Objectives
The present study aimed to investigate the differential diagnostic value of spectral CT parameters combined with enhanced CT imaging features in detecting mass-like lesions of the lungs.
3. Patients and Methods
3.1. Patients
In this case-control study, the malignant group consisted of malignant cases, and the benign group consisted of the controls. There were 358 suspected cases, collected from November 2021 to May 2022 in Jingzhou Hospital, affiliated to Yangtze University, Hubei, China. Pathological examinations were performed on 75 patients who met the inclusion criteria, including 22 benign and 53 malignant cases. Two patients without pathological examination recovered using an anti-inflammatory treatment, and there was no recurrence (clinically confirmed inflammatory lesions) during the six-month follow-up. The benign group mainly included cases of pneumonia, inflammatory pseudotumor, tuberculosis, granulomatous inflammation, and cryptogenic organizing pneumonia. On the other hand, the malignant group included cases of adenocarcinoma, squamous cell carcinoma, and neuroendocrine tumors.
The inclusion criteria were as follows: (1) detection of mass-like lung lesions without any drug or surgical treatments before examination; (2) no history of adverse reactions to iodine contrast; (3) no history of hyperthyroidism, severe liver disorder, or renal insufficiency; (4) cases confirmed by surgery or aspiration pathology, as well as inflammatory cases observed in clinical follow-ups for lesion size reduction or disappearance; and (5) ability to cooperate with spectral CT imaging in the gemstone spectral imaging (GSI) mode. The image quality met the requirements for diagnosis and reconstruction.
This study was approved by the Medical Ethics Committee of Jingzhou Hospital, affiliated to Yangtze University (protocol number: 2022-073-01).
3.2. Image Acquisition
All the patients were examined in the GSI mode of the GE Revolution 256 CT scanner. The parameters were set as follows: Rotation time, 0.6 seconds; tube current, 600 mA; tube voltage, 80 kV/140 kV (instantaneous switching); and reconstruction layer thickness, 0.9 mm/0.9 mm. The entire lung field was swept from the first rib to the level of the diaphragm. Through the median elbow vein, 300 mg/mL of Ioversol was injected using a high-pressure syringe at a dose of 1.5 mL/kg (flow rate, 3 mL/sec), followed by the injection of 30 mL of saline at the same flow rate; the scan delay time after contrast injection was 30 and 60 seconds, respectively.
3.3. Image Feature Analysis and Quantitative Measurement of DECT Data
Two senior physicians jointly reviewed the films and measured the quantitative parameters using the GSI Viewer software, without any knowledge of the clinical and pathological findings. They analyzed the enhanced CT features of each lesion, including the tumor location, maximum cross-sectional lesion diameter, presence of uniform enhancement, internal structures (e.g., air bronchogram sign and cavity), and the surrounding structures. Regions of interest (ROI) was drawn to encompass more than two-thirds of the solid component of the lesion, avoiding vessels, calcification, or necrotic changes. The ROI data were measured at three adjacent image levels, and then, the mean value was calculated. Besides, the Zeff value was measured on the 40- and 70-keV monochromatic images in the arterial phase (AP) and venous phase (VP). The slope of the spectral curve between 40 keV and 70 keV was also calculated ([Hu40keV-Hu70keV]/30). The IC was measured according to the iodine map at the arterial and venous ROIs, and the normalized iodine concentration (NIC) was calculated based on the pulmonary artery IC at the same level of the lesion as the standard.
3.4. Statistical Analysis
All collected data were analyzed in SPSS Version 25.0 (IBM SPSS Statistics for Windows, released in 2017, IBM Corp., Armonk, NY, USA). Normally distributed quantitative data are expressed as mean ± standard deviation (SD), while non-normally distributed data are expressed as median ± interquartile range (IQR). Categorical data in both groups were analyzed using chi-square test, and continuous variables were compared using student’s t-test (data with a normal distribution) and Mann-Whitney U test (data without a normal distribution), respectively. Additionally, a multifactorial logistic regression analysis was performed to determine the efficacy of CT features alone and CT features combined with spectral CT indices as independent predictors and to identify the optimal regression model for distinguishing benign and malignant lesions based on the area under the curve (AUC). Differences were considered statistically significant at a two-tailed P-value below 0.05.
4. Results
4.1. Patients
The clinical data of the patients are summarized in Table 1.
| Variables | Benign group (n = 24) | Malignant group (n = 53) | P-value |
|---|---|---|---|
| Age | 60.13 ± 11.64 c | 67.40 ± 8.30 c | 0.04 |
| Sex | |||
| Male | 20 | 37 | 0.21 |
| Female | 4 | 16 | |
| Smoking history | 15 | 23 | 0.12 |
| Clinical symptoms | |||
| Fever | 7 | 1 | 0.001 |
| Cough | 16 | 36 | 0.913 |
| Coughing sputum | 10 | 28 | 0.364 |
| Chest pain | 4 | 14 | 0.349 |
| Laboratory tests | |||
| Elevated leukocyte count | 4 | 6 | 0.518 |
| Elevated C-reactive protein level (n1 = 23, n2 = 31) | 7 | 11 | 0.192 |
a n1, Number of cases undergoing C-reactive protein tests in the benign group.
b n2, Number of cases undergoing C-reactive protein tests in the malignant group.
c Mean ± standard deviation.
4.2. Conventional CT Characteristics of the Patients in the Benign and Malignant Groups
According to the univariate analysis, some CT features were significantly different between the malignant and benign groups, including the long-axis diameter (44.86 ± 18.42 in the malignant group vs. 55.59 ± 22.57 in the benign group, P = 0.07), thickened adjacent vessel (58.33% in the benign group vs. 5.66% in the malignant group, P = 0.001), pleural retraction (29.17% in the benign group vs. 69.81% in the malignant group, P = 0.001), mediastinal lymphadenopathy (25.00% in the benign group vs. 62.26% in the malignant group, P = 0.02), mediastinal lymph node confluence (4.17% in the benign group vs. 41.51% in the malignant group, P = 0.01), and lesion diameter-mediastinum relationship (parallel relationship: 58.33% in the benign group vs. 20.75.% in the malignant group, P = 0.001) (Table 2).
| CT characteristics | Benign group (n = 24) | Malignant group (n = 53) | OR (95% CI) | t/χ2 | P-value |
|---|---|---|---|---|---|
| Location | |||||
| Upper lobe | 11 | 35 | 0.435 (0.163 - 1.164) | 2.804 | 0.094 |
| Middle and lower lobes | 13 | 18 | |||
| Long-axis diameter (mm) | 44.86 ± 18.42 a | 55.59 ± 22.57 b | -2.683 | 0.007 | |
| Internal | |||||
| Air space | 7 | 13 | 1.267 (0.430 - 3.731) | 0.185 | 0.667 |
| Air bronchogram | 1 | 2 | 1.109 (0.096 - 12.853) | 0.007 | 0.934 |
| Enhancement heterogeneity | |||||
| Homogeneous | 4 | 4 | - 0.374 | 0.708 | |
| Slightly or moderately heterogeneous | 6 | 13 | |||
| Marked heterogeneity | 14 | 26 | |||
| Surrounding structure | |||||
| Thickened adjacent vessel | 14 | 3 | 23.333 (5.641 - 96.514) | 26.641 | 0.001 |
| Peripheral emphysema | 4 | 20 | 0.330 (0.099 - 1.105) | 3.418 | 0.064 |
| Peripheral fibrosis | 24 | 50 | 1.060 (0.992 - 1.132) | 1.414 | 0.234 |
| Lesion margin-pleura relationship | |||||
| Wide based | 11 | 16 | 1.957 (0.724 - 5.289) | 1.776 | 0.183 |
| Narrow based | 13 | 37 | |||
| Pleural retraction | 7 | 37 | 0.178 (0.062 - 0.513) | 11.143 | 0.001 |
| Lesion diameter-mediastinum relationship | |||||
| Parallel | 14 | 11 | 5.345 (1.874 - 15.252) | 10.639 | 0.001 |
| Vertical | 10 | 42 | |||
| Mediastinal lymphadenopathy | 6 | 33 | 0.202 (0.069 - 0.594) | 9.177 | 0.002 |
| Mediastinal lymph node fusion | 1 | 22 | 0.061 (0.008-0.488) | 10.997 | 0.001 |
| Pleural effusion | 2 | 10 | 0.391 (0.079 - 1.942) | 1.394 | 0.238 |
Abbreviations: OR, odds ratio; CI, confidence interval.
a Median ± interquartile range.
b Mean ± standard deviation.
4.3. Comparison of Gemstone Spectral Imaging Parameters
Comparison of quantitative spectral CT parameters between the two groups is shown in Table 3.
| Variables | CT values at 40 keV | CT values at 70 keV | IC | NIC | K40-70 keV | Zeff |
|---|---|---|---|---|---|---|
| Arterial phase | ||||||
| Benign group (n = 24) | 157.25 ± 79.23 b | 79.63 ± 15.17 c | 14.73±10.65 b | 0.14 ± 0.10 b | 2.76 ± 2.05 b | 8.46 ± 0.63 b |
| Malignant group (n = 53) | 148.46 ± 25.36 c | 72.80 ± 10.63 c | 13.44 ± 3.24 c | 0.12 ± 0.05 b | 2.52 ± 0.60 c | 8.43 ± 0.28 c |
| T | 1.991 | 1.997 | 2.068 | 2.442 | 2.051 | 2.117 |
| P-value | 0.047 | 0.054 | 0.039 | 0.015 | 0.04 | 0.034 |
| Venous phase | ||||||
| Benign group (n = 24) | 176.45 ± 40.50 c | 81.93 ± 15.62 c | 17.52 ± 5.29 c | 0.41 ± 0.11 c | 3.15 ± 1.26 c | 8.60 ± 0.29 c |
| Malignant group (n = 53) | 158.42 ± 36.72 c | 78.50 ± 16.45 c | 13.87 ± 5.81 c | 0.35 ± 0.10 c | 2.76 ± 1.05 b | 8.39 ± 0.33 c |
| T | 1.784 | 1.626 | 2.149 | 2.433 | 1.199 | 2.141 |
| P-value | 0.078 | 0.108 | 0.035 | 0.017 | 0.231 | 0.035 |
Abbreviations: IC, iodine concentration; NIC, normalized iodine concentration.
a Zeff: Effective atomic number.
b Median ± interquartile range.
c Mean ± standard deviation.
Some spectral CT parameters in AP were significantly higher in the benign group compared to the malignant group, including CT values in 40 keV (157.25 ± 79.23 vs. 148.46 ± 25.36, P = 0.047) and K40-70 keV (2.76 ± 2.05 vs. 2.52 ± 0.60, P = 0.04). Besides, the IC (14.73 ± 10.65 vs. 13.44 ± 3.24, P = 0.039; 17.52 ± 5.29 vs. 13.87 ± 5.81, P = 0.035), NIC (0.15 ± 0.06 vs. 0.11 ± 0.05, P = 0.015; 0.41 ± 0.11 vs. 0.35 ± 0.10, P = 0.017), and Zeff (8.46 ± 0.63 vs. 8.43 ± 0.28, P = 0.034; 8.60 ± 0.29 vs. 8.39 ± 0.33, P = 0.035) in the benign group were significantly higher than those of the malignant group, both in the AP and VP. Figures 1 and 2 show the post-processed images of typical benign and malignant cases, respectively.
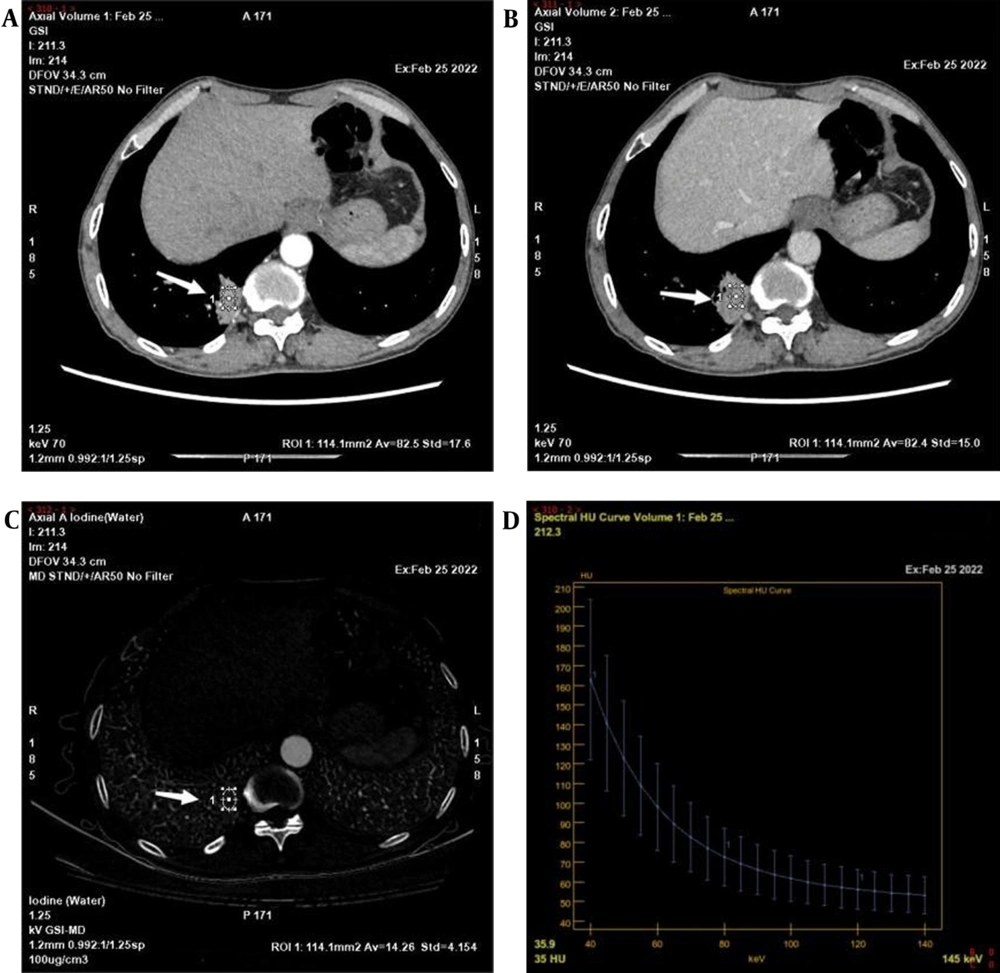
A-D, A 58-year-old man with granulomatous inflammation of the right lower lung, indicated by a white arrows. A-B, A 70-keV single-energy image in the arteriovenous phase; a mass-like high-density shadow can be seen in the right lower lobe of the lung next to the spine with adjacent bronchial occlusion. C, Iodine map in the arteriovenous phase; the iodine concentration (IC) of the lesion in the arteriovenous phase is 14.26 μg/cm3. D, Arterial phase energy spectral graph.
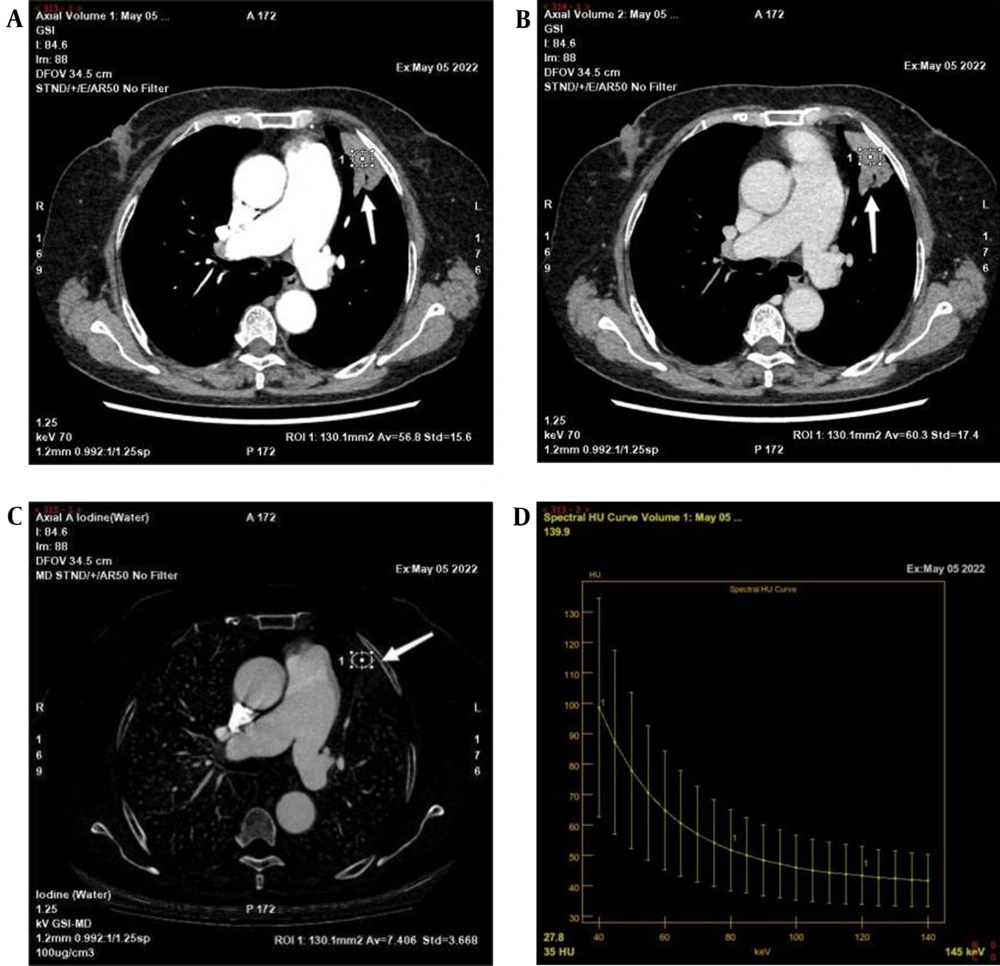
A-D, A 66-year-old man with adenocarcinoma of the left upper lung, indicated by a white arrows. A-B, A 70-keV single-energy image in the arteriovenous phase; a mass shadow of the left upper lobe of the lung can be seen with a cavity within it. C, An iodine base image in the arterial phase; the iodine concentration (IC) of the lesion in the arterial phase is 7.406 μg/cm3. D, Arterial phase energy spectral graph.
4.4. Multivariate Logistic Regression Analysis
According to the multivariate logistic regression analysis, the model of morphological characteristics included the lesion diameter, adjacent vessel thickening, and pleural depression sign, with a Cox-Snell R2 value of 0.485. The model of quantitative spectral CT parameters included the effective atomic number in the arterial phase, with a Cox-Snell R2 value of 0.139. Additionally, the combined model included IC in the arterial phase, effective atomic number in the arteriovenous phase, adjacent vessel thickening, pleural depression sign, lesion-mediastinum relationship, and mediastinal lymph node fusion, with a Cox-Snell R2 value of 0.536.
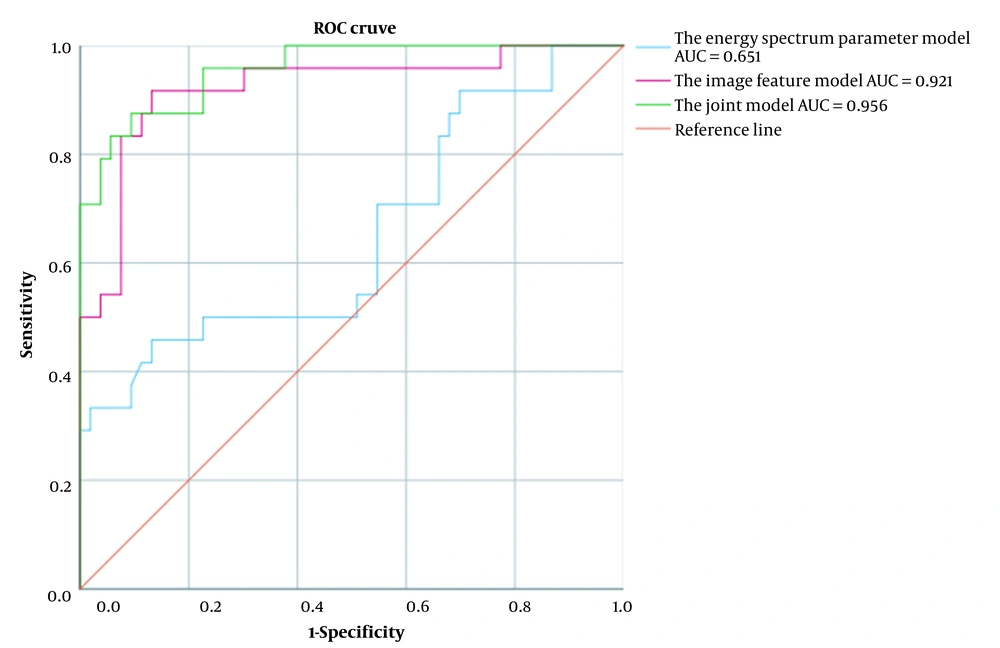
The efficacy of each model in the differential diagnosis of benign and malignant pulmonary mass-like lesions was examined by the ROC curve analysis, the results of which are shown in Table 4. Except for the image feature model with the highest sensitivity, the AUC and specificity of the combined model were higher than the other two groups, and its diagnostic performance was relatively good (Table 4 and Figure 3).
| Variables | AUC | 95% CI | P-value | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|---|---|
| Image feature model | 0.921 | 0.849 - 0.994 | 0.001 | 0.917 | 0.868 | 0.939 | 0.826 |
| Energy spectral CT parameter model | 0.651 | 0.510 - 0.793 | 0.034 | 0.458 | 0.868 | 0.885 | 0.420 |
| Combined model | 0.956 | 0.912 - 1.000 | 0.001 | 0.875 | 0.906 | 0.954 | 0.766 |
Abbreviations: PPV, positive predictive value; NPV, negative predictive value; AUC, area under the curve; CI, confidence interval.
5. Discussion
As the method of choice for lung cancer screening, CT examination of the chest indicates the morphological features of lesions and enhancement patterns, which are of great importance in clinical practice. The results of previous studies have shown that virtual monoenergetic or monochromatic imaging (VMI) using spectral CT substance-separation techniques can replace conventional CT imaging to some extent, while reducing the radiation dose (5). With the application of spectral CT in life sciences, post-processing techniques can be employed in addition to morphological parameters to determine quantitative parameters and other clinical diagnostic indices, which provide further information about the lesions.
The present study showed that adjacent vessel thickening was more frequently combined with other signs in the benign group compared to the malignant group, based on the univariate analysis of imaging features. The benign group in the current study mainly included inflammatory lesions. Inflammatory mediators, such as inflammatory cytokines, chemokines, and vasoactive amines released from inflammatory tissues, may increase vascular permeability and contribute to vasodilatation. Although infiltrating cells are different in acute and chronic inflammations, the persistence of inflammatory factors is common in both (6). The accelerated tissue growth and metabolism of tumor lesions, continuous cell proliferation, and increased oxygen demand and blood supply requirement can lead to the thickening of blood supply arteries. With the invasion of tumor cells into the vessel wall, leading to luminal narrowing or even occlusion, the blood supply is less abundant than that of inflammatory lesions.
In the current study, most peripheral lung cancers were associated with adjacent pleural retraction due to fibrous tissue contraction within the lesion, although several inflammatory groups could also cause pleural reactions due to inflammatory exudation, resulting in localized pleural thickening and adhesion. Pleural retraction was more common in the malignant group; therefore, it might be used as a useful marker for distinguishing benign and malignant mass-like lesions of the lungs. Although some imaging manifestations of the two groups overlapped, some characteristic CT manifestations that could help with the differential diagnosis included the larger diameter of the lesion, proximity of pleural retraction, perpendicularity of the lesion to the mediastinum, and combination of mediastinal lymph node enlargement with mediastinal lymph node confluence, which often suggested lung cancer. The AUC in the multivariate logistic regression analysis was 0.921, which indicated the clinical value of enhanced CT imaging parameters in the differential diagnosis of benign and malignant mass-like lesions of the lungs.
The most significant advantage of spectral CT over conventional CT is the possibility of obtaining single-energy virtual images and substance-separation images through post-processing (7). By using iodine-water as the base material pair, the iodine maps are plotted using the substance-separation technique to determine the tissue IC, which can provide a more objective and quantitative analysis of the lesion components. The results of this study showed that CT values at 40 keV and K40-70 keV in the AP phase and IC, NIC, and Zeff values in the arteriovenous phase were higher in the benign group compared to the malignant group (P < 0.05); this finding is consistent with the results of a study by Hou et al. (8). Iodine, as the main component of the contrast agent, can reflect the tissue hemodynamic state; therefore, it is a more sensitive and realistic index for reflecting the degree of tissue enhancement compared to CT values. Besides, NIC can reduce individual differences caused by differences in the contrast flow rate and individual circulation and is more accurate than IC (9).
The blood supply to inflammatory lesions differs from cancerous lesions, as blood is supplied by both pulmonary and bronchial arteries. Second, inflammatory mediators released from inflammatory lesions can cause peripheral vasodilation, increase vascular permeability and edema in the adjacent tissues, and significantly increase perfusion in the lesion. Third, bronchial dilation near inflammatory lesions in the chronic phase is more pronounced than lung cancer. Overall, these factors can cause the inflamed tissue to have a better blood supply than cancerous lesions (10). Consistent with previous reports in the literature, suggesting that the slope of the energy spectrum curve in the AP and VP is significantly higher in inflammatory lung lesions compared to lung cancers (11), the energy spectral curve describes the attenuation of material under different energy X-ray irradiations and is closely related to the material density. The slope of the energy spectral curve in the AP and VP indicates that the blood supply of benign lung lesions is different from that of the malignant group, based on the quantitative analysis (12). The results of this study suggest that spectral CT parameters can be used as reliable indicators for diagnosis of inflammatory diseases and cancers.
To further validate the diagnostic value of quantitative spectral CT parameters in the diagnosis of benign and malignant pulmonary mass-like lesions, a combined model was established by using these parameters along with morphological CT features. The results showed that the AUC of the combined diagnostic model was higher than that of the CT features model. The diagnostic performance of the combined model was superior, which might help improve the diagnostic accuracy.
This study had some limitations. First, the sample size was small, especially the number of benign cases; therefore, further data collection is needed in the future. Second, mass-like lesions of different sizes were not subdivided in this study, and different tumor stages were not analyzed in further detail. Overall, the sample size needs to be increased for a more comprehensive analysis in follow-up studies.
In conclusion, compared to the CT features alone, the combination of CT features with quantitative spectral CT parameters showed a superior diagnostic performance. This combination was a powerful predictive index for the differential diagnosis of pulmonary mass-like lesions, which can non-invasively provide additional information for the preoperative therapeutic evaluation of patients with lung diseases and help clinicians select the best treatment plan.