1. Background
Susceptibility weighted imaging (SWI) is the unique technique to display cerebral veins in vivo by using deoxyhemoglobin as an endogenous contrast agent (1). In recent years, researchers have paid more attention on alterations of deep medullary veins (DMVs) and their clinical value in patients with acute cerebral ischemic infarction by using SWI. Studies have demonstrated that prominent DMVs were correlated to decreased cerebral blood flow, as well as clinical outcome (2-4), and suggested as a bio-marker of severity and prognosis for patients with acute ischemic infarction.
However, little is known whether alterations of DMVs also exist in transient ischemic attack (TIA) patients. As we know, TIA and cerebral infarction share the same risk factors and mechanisms. Up to 23% of cerebral infarctions are preceded from TIA (5). Moreover, previous studies indicated that a hypoperfused lesion or hemisphere was detected by perfusion imaging, even though TIA onset is a transient episode. Thus, we hypothesize that DMVs changes would also be identified in TIA patients by using SWI.
2. Objectives
In this study, we aim to describe and interpret the alteration of DMVs in TIA patients by assessing the asymmetry and score of DMVs.
3. Patients and Methods
3.1. Patients
Fifty-three patients with classic TIA, and 53 controls with matched age (control group age = TIA patient age ± 5 years old, for example: a 45 year-old TIA patient vs. a control with age ranging from 40 to 50 years) and gender (for example: a female TIA vs. a female control) were recruited at our hospital from January 2014 to October 2015. All participants were provided written informed consent prior to participation in this study. The study was approved by the institutional review board of our hospital.
Inclusion criteria for TIA group: a, according to the definition recommended by American heart association/American stroke association (AHA/ASA) in 2009: patients with a transient episode of neurological dysfunction caused by focal ischemia, without acute infarction evidence (5); b, negatively restricted diffusion on DWI; c, no stroke, TIA or heart disease history; d, with unilateral motor or somatosensory dysfunction.
Inclusion criteria for controls: a, no clinical symptom or complaints; b, no positive findings except a little lacunar cerebral infarction in the brain.
Exclusion criteria: a, abnormal findings on brain MRI such as space occupying lesions, head trauma evidence, hemorrhage, infarction or infection; b, taking hormone and vascular dilation drugs; c, contraindications for MR scan; d, poor image quality.
3.2. MRI Protocol
All subjects were imaged within 48 hours from onset with multi-modal MR imaging protocol, including T1WI, T2WI, fluid attenuated inversion recovery (FLAIR), diffusion weighted imaging (DWI) and SWI sequence on a 3.0T MRI system (Discovery MR750,GE,USA) equipped with an eight-channel phased array head coil. Axial T1WI, T2WI, FLAIR and DWI sequence was used to except the lesions such as acute infarction, infection or trauma. The SWI sequence was in an axial orientation parallel to the anterior commissure to posterior commissure line and covered the whole lateral ventricles with the following relevant parameters: repetition time (TR) = 27 ms; echo time (TE) = 20 ms; flip angle = 15°; slice thickness = 2 mm; intersection gap = 0 mm; field of view = 24 × 24 cm2; matrix number = 256 × 256.
3.3. Imaging Analysis
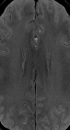
All of the SWI images were reconstructed through minimal intensity projection at a workstation (ADW4.6, GE), and reviewed by three neuroradiologists. Disagreements were resolved by consensus (Dr. Yang Duan, Dr. Benqiang Yang, and Dr. Zhihua Xu). To quantify the visibility and prominence of DMVs, a scoring system was applied (3). Bilateral hemisphere was assessed separately from score 0 to 3 as shown in Figure 1 (0, not visible; 1, faint visibility; 2, unequivocal visibility and 3, very prominent). A different score for bilateral hemispheres was defined as asymmetric DMVs (ADMVs), and an equal score was defined as symmetric DMVs. The higher score of bilateral hemispheres was regarded as its DMVs score value in ADMVs group. In ADMVs, neurologists (Dr. Huisheng Chen, Dr. Cheng Xia) defined both affected and non-affected hemisphere according to clinical symptom. Based on whether the affected hemisphere gets a higher score or not, we defined it as ipsilateral or contralateral asymmetric DMVs (iADMVs or cADMVs) (Figure 1C and D).
Scoring system for quantification of deep medullary veins (DMVs). A, 0: Not visible (one control, 56 -year-old male); B, 1: Faintly visible (symmetric DMVs: transient ischemic attack (TIA) patient, 34-year-old female with right limb weakness for 8 minutes); C, 2: Unequivocally visible (contralateral prominent DMVs: TIA patient, 78 -year-old female with left hemiparesis for 35 minutes); D, 3: Very prominent (ipsilateral prominent DMVs: TIA patient, 56-year-old female with left arm and leg weakness for 9 minutes).
3.4. Statistical Analysis
The data were analyzed by using Statistical Package for Social Sciences (SPSS) software for windows (IBM Corp., Released 2011, IBM SPSS Statistics for Windows, Ver. 20.0, Armonk NY: IBM Corp). Inter-observer agreement was assessed using Kappa statistics (κ). κ > 0.6 is considered good agreement, while κ > 0.8 is considered excellent (6). The score of DMVs were used as an ordinal categorical variable. The asymmetry and score of DMVs on SWI in patients with TIA was compared with control group using McNemar’s test, and Wilcoxion signed-rank test respectively.
4. Results
Fifty-three patients (male: 29/53, mean age: 61.74 ± 13.55 years; range: 35 to 86 years) and 53 age and gender matched controls (male: 29/53, mean age: 61.35 ± 13.20 years; range: 34 to 87 years) were included in this study (Table 1). The agreement between neuroradiologists for asymmetry of DMVs on SWI in both groups were excellent (TIA group: κ = 0.851; control group: 0.900), as well as score of DMVs (κ = 0.841, 0.867 respectively).
Of the fifty-three patients, 16 patients were present with ADMVs, including 10 iADMVs, and six cADMVs. There were statistically significant differences for the asymmetry of DMVs between TIA and the control group (P < 0.05), as well as DMVs score (Table 1), even when they were analyzed by further stratification analysis according to the asymmetry of DMVs (asymmetric group: P = 0.003; symmetric group: P = 0.001) (Figure 2).
| Variables | TIA Group (N = 53) | Control Group (N = 53) | P Value |
|---|---|---|---|
| Gender | |||
| Male | 29 | 29 | |
| Female | 24 | 24 | |
| Age, y (mean ± SD) | 61.74 ± 13.55 | 61.35 ± 13.20 | |
| Asymmetry | < 0.001 | ||
| Symmetric | 37 | 52 | |
| Asymmetric | 16 | 1 | |
| DMVs score | < 0.001 | ||
| 0 | 7 | 12 | |
| 1 | 15 | 26 | |
| 2 | 21 | 15 | |
| 3 | 10 | 0 |
Clinical Data, Asymmetry and Score of Deep Medullary Veins (DMVs) in Transient Ischemic Attack (TIA) and Control Groupa
The box plot of deep medullary veins (DMVs) score for both transient ischemic attack (TIA) and control group. DMVs score has significant difference with stratification analysis based on the asymmetry of DMVs between TIA and control group (asymmetric group: P = 0.003; symmetric group: P = 0.001).
5. Discussion
In this study, results indicate that the agreement of asymmetry and score of DMVs were excellent between neuroradiologists (inter-rater) by using SWI. We found that the visibility of DMVs in patients with TIA was increased, with or without ADMVs, including iADMVs and cADMVs.
As we have known, DMVs directly participate the drainage of white matter via the subependymal veins into the internal cerebral vein or the basal vein of Rosenthal (7). Researchers have demonstrated that DMVs have relative clinical significance for stroke patients. The initial discovery in acute stroke patients on ipsilateral prominent DMVs, which was called the “brush sign”, was made by Morita et al. in 2008 and was rapidly confirmed (8) by others. Subsequent studies have shown that the presence of ipsilateral or contralateral prominent DMVs in patients with acute or subacute stroke has a relationship with hemodynamic alteration, as well as clinical outcome. Han et al. found that ADMVs have a negative correlation with cerebrovascular reactivity (2). Mucke et al. also found that ADMVs in acute cerebral infarction on SWI was correlated with poor outcome (3). In this study, alterations of DMVs in patients with TIA were studied for the first time by using SWI, and we try to interpret them.
We assume that the increased visibility of DMVs on SWI is caused by two possible mechanisms: one is an increased venous volume because of regional ischemia and leads to vasodilation (9, 10); and the other is that the ratio of deoxyhemoglobin to oxyhemoglobin is increased in the hypoperfused tissue due to an uncoupling between oxygen supply and demand (11). Thus, neuroradiologists are able to identify DMVs on SWI with excellent agreement for the structure and signal change.
A lot of studies have confirmed that TIA patients are often found with a lesion with hypoperfusion or decreased blood flow (12-14). Thus, the decreased blood flow gives rise to a relative increase of oxygen extraction fraction (OEF) spontaneously within the cerebral tissue at risk (15). These changes increased deoxyhemoglobin concentration in the venous system, and then susceptibility changes lead to iADMVs on SWI (16). This result is consistent with previous studies on OEF changes after TIA by using positron emission tomography (PET) (17).
Moreover, cADMVs are observed in this study. These DMV changes with ipsilateral hyperperfusion were reported on subacute stroke by Yu et al. (4) in 2016. We think that cADMVs reflect a collateral blood flow established spontaneously through Willis’ circle or leptomeningeal vessels, resulting in a decreased concentration of deoxyhemoglobin in the draining veins because of excessive oxygen delivery and thus a more prominent hypointensity within the DMVs.
The alterations of DMVs in TIA patients were not only increased visibility of DMVs, but also presented with or without ADMVs. These may indicate that although focal cerebral blood flow reduced in TIA patients, the whole brain perfusion changed for compensation. On the other hand, we assume that cerebral ischemia is a dynamic process, and iADMVs, cADMVs, and symmetric DMVs may demonstrate changes of a different stage or phase during the ischemia process in TIA. cADMVs may indicate an effective compensation, while iADMVs may indicate a relative decompensation for blood flow. Symmetric DMVs with increased visibility may reflect chronic ischemia, which may share the mechanism in leukoaraiosis demonstrated by Yan et al. (18).
Above all, whatever the mechanism is, we think the increased visibility DMVs with or without ADMVs would give us additional information for understanding the hemodynamic alteration of TIA. Furthermore, due to DMVs easily identified and well qualified on 3.0 T SWI, it is worth having a further study in the future.
There are limitations to this study. First, it had relatively small samples. A large number of case studies should be included at multiple centers in the future. Second, our results were lack of the perfusion image, because more MR protocols (for example, arterial spin labeling [ASL] perfusion MRI) were not approved by the institutional review board. Third, the evaluation of DMVs’ appearance is based on the degree of visibility by the neuroradiologist’s naked vision, which may lead to bias for quantification of DMVs. With the development of quantitative susceptibility mapping (QSM), it may help improve the accuracy for understanding the change of DMVs correlated with TIA by analyzing the oxygenation level of cerebral veins through conversion of susceptibility values within the veins to oxygen saturation (19). Finally, our follow up was not continued for a long time. A future study should perform to focus on whether longitudinal changes of DMV would have any correlation with the risk for stroke after TIA.
In conclusion, based on asymmetry and score of DMVs, the visibility of DMVs in patients with TIA increased, with or without ADMVs, and these alterations may reflect hemodynamic information following TIA process, laying foundations for DMVs application in TIA.