1. Background
Tonsillar herniation is a disease that develops in the metencephalon, progresses from the foramen magnum, and is displaced toward the cervical vertebral canal (1, 2). Although this condition is known primarily to be a congenital malformation, acquired causes also play a role. Its etiology has been attributed to several causes including hydrodynamic factors, traction, neuroschisis, and primary mesodermal failure. Tonsillar herniation may also result from acquired pathologies that emerge due to the circulation of cerebrospinal fluid (CSF), as well as intracranial lesions and connective tissue disorders. Its etiology is still not fully understood (3-8).
It is proposed that a decrease in the volume of the posterior cranial fossa may play an active role in the formation of Chiari malformation type I (CMI). This decrease results from insufficient development of the bones that comprise the posterior cranial fossa; alternatively, the decrease may be due to the size of the organs it contains (1, 9, 10).
The prevalence of CMI may be difficult to estimate because it is asymptomatic in many cases. However, Meadows et al. have reported its prevalence to be about 0.75% of the population (11).
The most common symptom of CMI is pain that can be reduced or increased by means of a Valsalva maneuver in the occipital and upper cervical region (12).
Because of the structures contained in the posterior cranial fossa, this is a crucial cavity in terms of the operations carried out within it. The cavity contains 10 pairs of cranial nerves, the mesencephalon, the pons, and the medulla oblongata. In addition, it is a route for CSF transition. Arterial veining is vital in terms of the structures it supplies and its adjuvant structures (13, 14).
2. Objectives
The objectives of this study are to measure the size of the posterior cranial fossa, the cerebrum, and the cerebellum using MRI images; to define values that can help to explain the morphology of these structures; to establish the incidence of tonsillar herniation; to reveal the possible association between these parameters and tonsillar herniation; and lastly, to shed light on the etiology of tonsillar herniation.
3. Patients and Methods
3.1. Study Group
The study included all cranial MR images relating to patients who applied to two hospitals (the radiology unit of Sivas Numune hospital and the radiology department of Cumhuriyet University Hospital) with headache complaints between January 2009 and July 2013. The exclusion criteria for the study were patients with disease affecting the bones and history of skull fracture. In all, 629 females and 423 males were included in the current study. The necessary approval was received from the Sivas local ethics committee (2013-07/23).
3.2. MR Protocol
Cranial MR images were acquired by means of cervical spinal coils using a 1.5 Tesla magnetic field power system (Excelart, Toshiba, Japan; Magnetom Symphony, Siemens, Erlangen, Germany). Through the SE T1-weighted images obtained from the sagittal plane [repetition time (TR): 550 msn; echo time (TE): 10 msn; flip angle (FA): 90/180; section thickness: 5 mm; matrix: 160 × 256], evaluations were performed based on the T1- and T2-weighted transverse and sagittal sections.
3.3. Imaging Analysis
We found the midsagittal plane by applying the criteria used by Allen et al. (15). The criteria confirming the midsagittal section were as follows: the sulcus corporis callosi separating the corpus callosum from the gyrus cinguli; the aqueductus cerebri between the tegmentum and the tectum; the presence of a visible “V” from the fornix of the fourth ventricle; and a non-visible hemispherium cerebelli.
In the midsagittal sections of the T1 images, we performed the same measurements that we carried out in the sagittal plane. In the sections where the cranium was widest in the T2 images, we then performed the same measurements that we carried out in the transverse plane. All of the morphometric measurements were performed using Syngo FastView software. We used various measurement points for the cerebrum, cerebellum, cranium, and cranial fossa. Specifically, the anthropological points below were taken as a reference on the sagittal and axial T1 and T2 images (16-18):
•Nasion: Intersection point of the sutura frontonasalis and the sutura internasalis.
•Glabella: The most salient point on the midsagittal plane between the arcus superciliaris ridges.
•Bregma: Intersection point of the sutura sagittalis and the coronalis.
•Vertex: Highest point of the head in the sagittal plane.
•Lambda: Intersection point of the sutura sagittalis and the lambdoidea.
•Opisthocranion: Most salient posterior point of the occiput.
•Euryon: Most salient laterally situated point of the os parietale.
•Basion: Midpoint of the anterior ledge of the foramen magnum.
•Opisthion: Midpoint of the posterior edge of the foramen magnum.
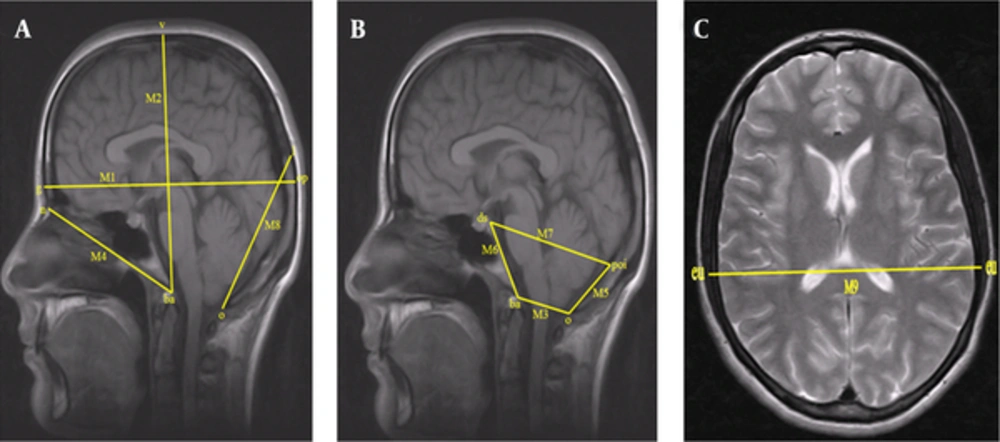
The following lengths were measured on the midsagittal T1-weighted images so that the sizes of the posterior cranial fossa and cranium could be evaluated (Figure 1A) (3, 4, 8, 19, 20).
M1: Distance between the glabella (g) and the opisthocranion (op) (maximum cranial length).
M2: Distance between the basion (ba) and the vertex (v) (maximum cranial height).
M3: Distance between the basion (ba) and the opisthion (o) (foramen magnum sagittal diameter).
M4: Distance between the nasion (n) and the basion (ba) (cranium base length).
M5: Distance between the opisthion (o) and the protuberentia occipitalis interna (poi) (supraocciput).
M6: Distance between the basion (b) and the dorsum sellae (ds) top edge (clivus length).
M7: Distance between the dorsum sellae (ds) and the protuberentia occipitalis interna (poi) (FCP anteroposterior length).
M8: Distance between the opisthion (o) and the lambda (l) (occipital cord length).
3.4. Cranium Width Measurements on T2 Axial Section (Figure 1B)
M9: Distance between the euryon (eu) and the euryon (eu) (maximum cranial width). (Figure 1C).
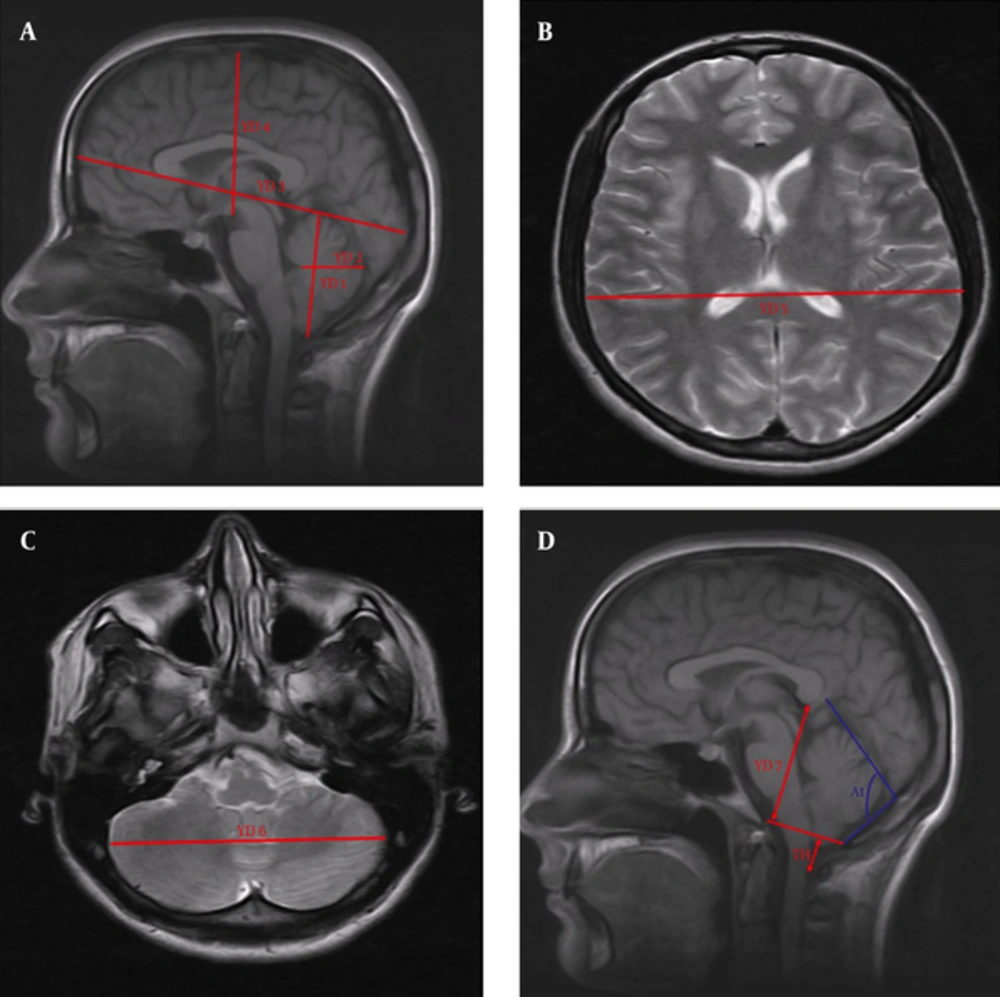
3.5. Measurements of the Cerebrum and Cerebellum on the T1 Midsagittal Section (Figure 2A)
YD1: Distance between the lowest and highest points of the cerebellum.
YD2: Distance between the most posterior point of the fourth ventricle and the most salient point of the posterior cerebellum.
YD3: Distance between the polus frontalis and the polus occipitalis (the longest anteroposterior diameter of the cerebrum).
YD4: Distance between the highest point of the cerebrum and the corpus mamillare.
3.6. Measurement of the Cerebrum on the T2 Axial Section (Figure 2B).
YD5: Lateral distance between the points most remote from each other in the cerebral hemispheres.
3.7. Measurement of the Cerebellum on the T2 Axial Section (Figure 2C)
YD6: Lateral distance between the points most remote from each other in the cerebellar hemispheres.
3.8. Measurements of the Tonsillar Herniation, the Posterior Height of the Cranial Fossa, and the Tentorial Slope on the T1 Midsagittal Section (Figure 2D)
YD7: Posterior height of the cranial fossa. Length of a line perpendicularly drawn from the inferior surface of the splenium corporis callosi to the foramen magnum plane (4, 21).
A1: Angle between the tentorium cerebelli and the supraocciput (tentorium cerebelli slope).
TH: We ascertained the extent of the tonsillar herniation by measuring the length of the tonsilla cerebelli, which remained under the line that was drawn between the basion and the opisthion. Herniations with a length of tonsilla cerebelli expanding from the level of the foramen magnum to the canalis vertebralis > 3 mm were considered to be CMI (22-25).
3.9. Statistical Analysis
Data obtained in this study were loaded in SPSS ver. 14 software (SPSS Inc., Chicago, Il, USA). The significance of the difference between two independent tests was used in cases where the parametric test assumptions were fulfilled; otherwise, the Mann-Whitney U test was used. Our results were expressed as the arithmetic mean ± standard deviation and P values > 0.05 were considered to be statistically significant.
4. Results
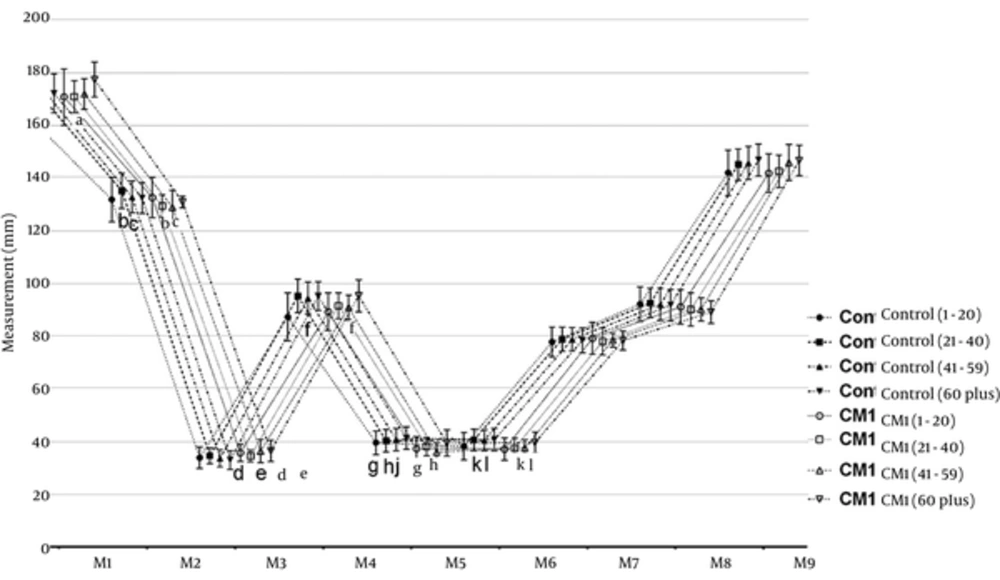
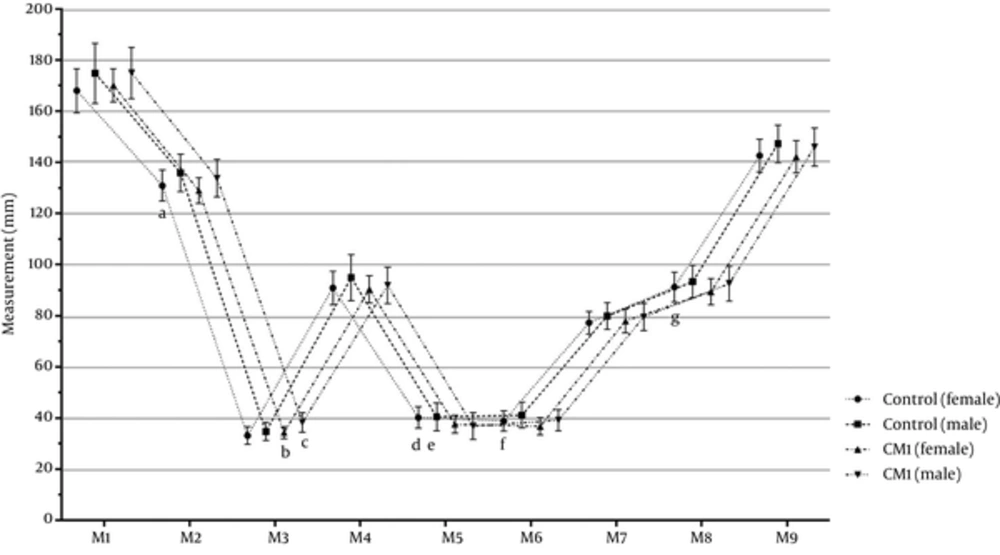
Figure 3 shows the cranial measurements of the age groups in the control and CMI groups. First, the M1 - M9 measurements of the CMI and control groups were compared and the participants then categorized based on age as 1 - 20, 21 - 40, 41 - 59, and 60 years or higher age groups. Overall, we found the following significant differences in the M1 - M9 measurements. In the CMI group, the M2, M5, and M6 measurements were significantly lower than those of the control group (P < 0.05). In the Arnold-Chiari type I group, M3 was significantly higher than in the control group (P < 0.05). Furthermore, the CMI and control groups were similar regarding to the M1, M4, M7, M8, and M9 measurements (P > 0.05).
Cranial measurements of age groups in the control and Arnold-Chiari type I groups; data were expressed as mean ± SD. CMI: Arnold-Chiari type I malformation; a, d, g P < 0.05 vs. control (1 - 20) subgroups; b, h, k P < 0.05 vs. control (21 - 40) subgroups; c, e, f, j, l P < 0.05 vs. control (41 - 59) subgroups.
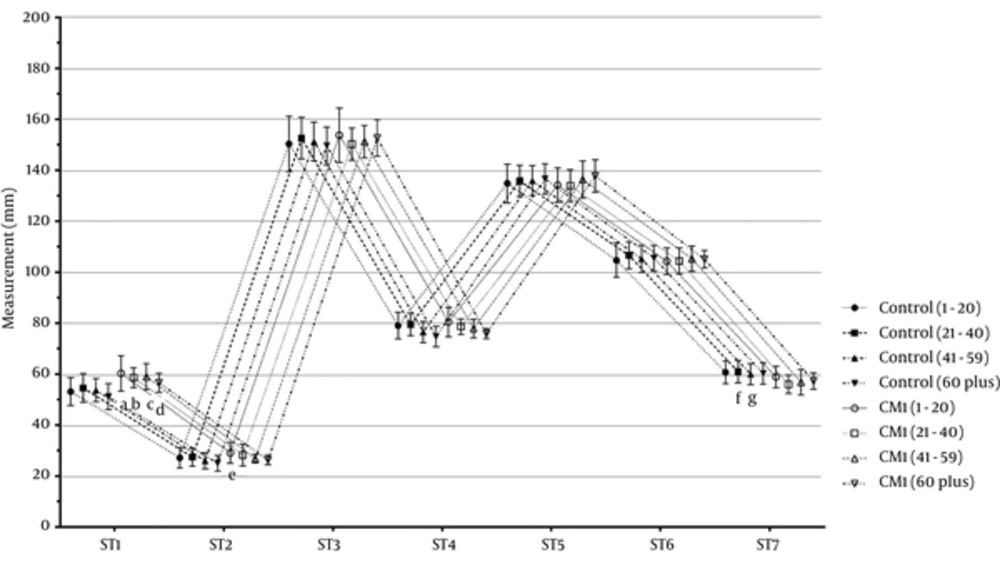
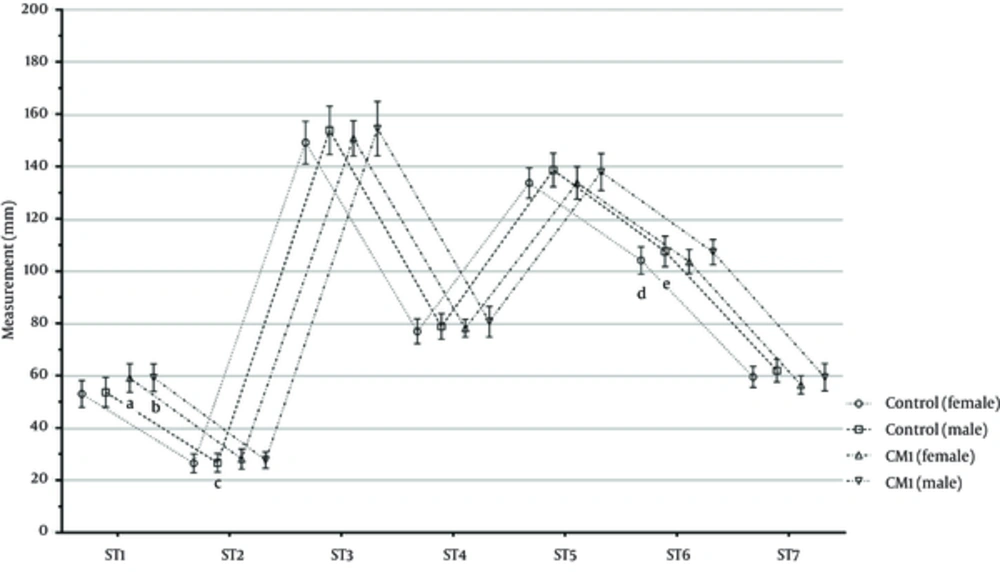
Figure 4 displays the cerebral and cerebellar measurements of all age groups in the CMI and control groups. First, the ST1 - ST7 measurements of the CMI and control groups were compared and the participants then categorized based on age as 1 - 20, 21 - 40, 41 - 59, and 60 years or higher age groups. Overall, we found the following significant differences in the ST1 - ST7 measurements. In the CMI group, the ST1 and ST2 measurements were significantly higher than those of the control group (P < 0.05). In the CMI group, the ST7 measurement was significantly lower than in the control group (P < 0.05). Furthermore, the CMI and control groups were similar regarding to the ST3, ST4, ST5, and ST6 measurements (P > 0.05).
Cerebral and cerebellar measurements of all age groups in the Arnold-Chiari type I and control groups; data were expressed as mean ± SD; CMI: Arnold-Chiari type I malformation; a, e P < 0.05 vs. CMI (1 - 20) subgroups; b, f P < 0.05 vs. control (21 - 40) group; c, g P < 0.05 vs. control 41 - 59 group; dP < 0.05 vs. control (60 plus) group.
Figure 5 shows the cranial measurements of the gender groups in the control and CMI groups. In the female CMI group, the M2, M5, M6, and M8 measurements were significantly higher than those in the female control group (P < 0.05); the M3 measurement of the female and male CMI groups were significantly higher than those of the female control groups (P < 0.05); and the M5 measurement of the female CMI group was significantly lower than in the female control group (P < 0.05). Furthermore, all groups were similar regarding to the M1, M4, M7, and M9 measurements (P > 0.05).
Figure 6 presents the cerebral and cerebellar measurements of the gender groups in the CMI and control groups. In the female CMI groups, the ST1 and ST2 measurements were significantly higher than those in the female control group (P < 0.05). In the female and male CMI groups, the ST7 measurements were significantly lower than those in the male control group (P < 0.05). In the male CMI group, the ST7 measurement was significantly lower than in the male control group (P < 0.05). Furthermore, the CMI and control groups were similar regarding to the ST3, ST4, ST5, and ST6 measurements (P > 0.05).
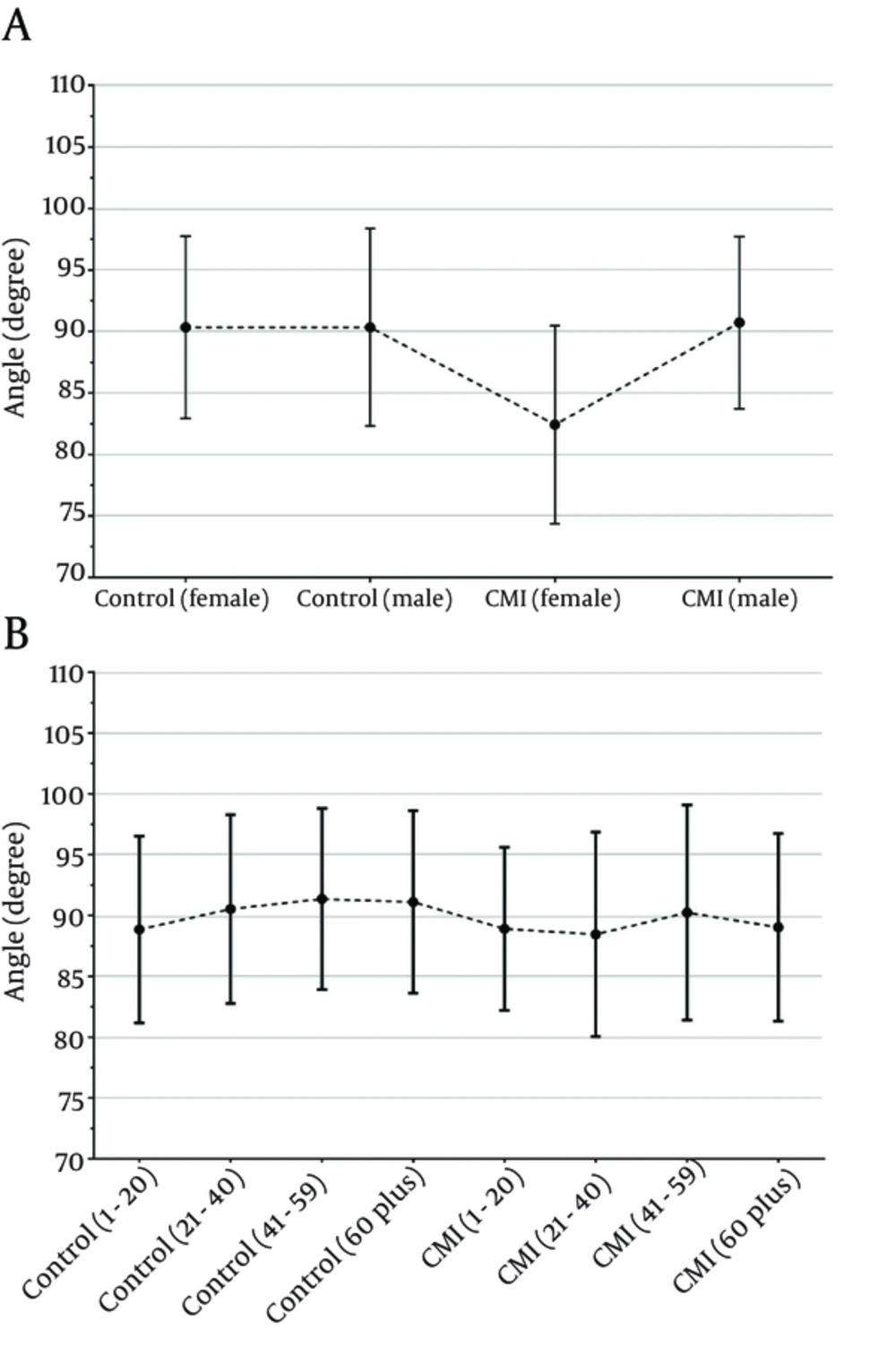
Figure 7 shows the degree of tentorial slope angle relating to the gender and age groups of the CMI and control groups. There was no significant difference between the study groups regarding to degree of tentorial slope (P > 0.05).
5. Discussion
The anteroposterior diameter of the foramen magnum was higher in the people who developed tonsillar herniation than in the healthy people. On the other hand, maximum cranial height, supraocciput length, and clivus length were lower in the patients with tonsillar herniation.
In numerous existing studies, the anteroposterior diameter of the foramen magnum was defined by measuring the distance between the basion and the opisthion (3, 20-22, 26, 27).
Dağtekin et al. reported that the foramen magnum diameter was found to be 40.1 mm in the CMI group and 32.4 mm in the controls, and that the difference was statistically significant (26).
Aydın et al. (21) found that the foramen magnum diameter was 31.7 ± 6.1 mm in the CMI group and 25.2 ± 3.8 mm in the controls, and that the difference was statistically significant. Based on their results, they proposed that an underdeveloped bony structure in the intrauterine phase causes the contents of the posterior fossa to become a downward hernia when the caudal metencephalon is normally developed. They also reported that this may lead to expansion of the anteroposterior diameter of the foramen magnum.
In contrast, Hwang et al. (27) found the diameter of the foramen to be 24.78 mm in the CMI patients and 29.54 mm in the controls. The authors reported that this difference was statistically significant.
Likewise, Leikola et al. (28) found that the diameter of the foramen magnum was smaller in pediatric patients with nonsyndromic craniosynostosis.
On the other hand, despite a larger diameter of the foramen magnum ın the CMI group, Karagoz et al. (20), Sekula et al. (3), and Milhorat et al. (29, 30) reported that the difference was not statistically significant.
In this study, we found that the anteroposterior diameter of the foramen magnum was 35.43 ± 3.34 mm in CMI patients and 33.74 ± 3.57 mm in the control group. The difference between the CMI and control groups was statistically significant (P = 0.001). Although the increase in the diameter of the foramen magnum that we found in our CMI patients showed similarities with the results of Dagtekin et al. (26) and Aydın et al. (21), Hwang et al. (27) and Leikola et al. (28) reported that the diameter of the foramen magnum was lower in the CMI patients. This difference may have resulted from the low number of patients, as noted by the authors. We also thought that this outcome may have been due to the fact that patients with craniosynostosis were included in the study by Leikola et al. (28).
In their study, Aydın et al. (21) referred to hypoplasia and the fact that, unlike the normal development of soft tissues, bony structures in the fossa may lead to herniation toward the cervical canal. Secondary to this, the diameter of the foramen magnum may be larger in CMI patients, as was found in our study.
One of the measurements required in order to elucidate the etiology of tonsillar herniation is the length of the supraocciput. This length was found to be smaller in CMI patients in the literature, with researchers reporting that the difference was statistically significant (4, 8, 20, 26, 29, 30). Sekula et al. (3), Aydın et al. (21), Furtado et al. (19), Hwang et al. (27), and Heiss et al. (31) did not report a statistically significant difference between the groups.
In this study, we found that the length of the supraocciput was 37.22 ± 4.17 mm in the CMI patients and 40.29 ± 4.79 mm in the controls. This difference between the patient and control groups was statistically significant (P = 0.001). A decrease in supraocciput length in the CMI patients might be attributed to occipital bone hypoplasia, which is a factor in the etiology of the condition.
Our other finding suggesting occipital bone hypoplasia in the CMI patients was the decrease in the length of the clivus. In our study, we found that the length of the clivus was 37.46 ± 3.75 mm in the CMI patients and 39.78 ± 4.62 mm in the controls. This difference between the patient and control groups was statistically significant (P = 0.001).
Like our outcomes, many authors reported that the length of the clivus was statistically significantly lower in the patients who developed herniation (3, 4, 20, 21, 27, 29, 31).
On the other hand, Nishikawa et al. (8) and Dagtekin et al. (26) found the clivus length shorter in people with tonsillar herniation, although they reported that the difference was not statistically significant.
In the literature, the anteroposterior diameter of the cranial fossa was found to be smaller in CMI patients (3, 20, 21).
Karagöz et al. proposed that this smaller length in CMI patients might lead to compensatory anterior growth of the tentorium (20).
In the current study, despite the fact that the anteroposterior length of the posterior fossa was slightly bigger in the CMI patients, this difference was not statistically significant.
Researchers who examined the change of the angle between the length of the supraocciput and the tentorium cerebelli line (tentorial angle) in the people with herniation found that this angle was greater than in the healthy individuals (3, 4, 26, 27).
In this study, we found that the tentorial angle was 89.08 ± 7.78o in the CMI patients and 90.33 ± 7.66o in the controls. This difference between the patient and control groups was not statistically significant (P = 0.209).
Dagtekin et al. (26) found that the mean length of the tonsillar herniation was 7.4 mm (4.9 - 12.1 mm). On the other hand, in a study by Aydın et al. (21), the mean quantity of the tonsillar herniation was found to be 12.6 mm (5 - 38 mm). Milhorat et al. found that tonsillar herniation was 9.8 ± 5.8 mm in the CMI patients and 2.1 ± 3.7 mm in the controls (P < 0.001) (4).
Elster and Chen (32) found that the degree of tonsillar herniation was higher in those having the findings of spinal cord, cerebrum and cerebral trunk.
Mikulis et al. (33) found that the mean extent of tonsillar herniation was 6 mm in the first decade, 5 mm between the second and third decades, 4 mm between the fourth and eighth decades, and 3 mm in the ninth decade. The authors suggested that there was a statistically significant decrease in the degree of the herniation with aging.
Aboulezz et al. (34) claimed that tonsillar herniation lower than 5 mm from the level of the foramen magnum was pathologic.
Barkovich et al. (23) reported that in the absence of syringomyelia, a herniation of 2 mm or less was clinically insignificant.
Urbizu et al. (25) used a tonsillar herniation greater than 3 mm on MRI as a base, and this resulted in several symptoms and findings (neural compression in the craniovertebral junction, syringomyelia, and cerebellar or intracranial hypertension) for the diagnosis of CMI. Using the results of that study, the authors supported the hypothesis that variability in the genes relating to the paraxial mesoderm might affect the size of the FCP, causing CMI.
Heiss et al. (31) found the mean value of tonsillar ectopia to be 12.3 mm (5 - 22.7 mm) before the patients were taken for the operation.
Milhorat et al. (30) defined herniation as tonsilla cerebelli lying more than 5 - 7 mm below the level of the foramen magnum; those authors described a prolapse of 0 - 4 mm as cerebellar tonsils.
Aitken et al. (35) described tonsillar herniation of between 2 and 4 mm as borderline herniation. In essence, 5 mm is considered to be a cut-off value for the overall majority in terms of tonsillar ectopia, although it has been reported that increasing symptoms and syringomyelia requiring surgical intervention have been seen in patients with lower degrees of herniation.
Sahuquillo et al. (24) defined CMI tonsilla cerebelli as sloping down at least 3 mm from the foramen magnum.
In this study, the mean amount of herniation was found to be 4.85 ± 3.09 mm.
Aydın et al. (21) found the height of the posterior fossa to be 124.7 ± 15.7 mm in the CMI group and 141.2 ± 6.8 mm in the control group; they also reported that this difference was not statistically significant.
In our study, we found the mean height of the posterior fossa to be 57.31 ± 4.21 mm in the CMI patients and 60.5 4 ± 4.30 mm in the controls. Additionally, we found the height of the posterior fossa statistically significantly smaller in the CMI patients compared to the controls. The reduction we found in the height of the FCP was similar to that found in the study by Aydın et al. (21). However, the difference between the means, despite the measurements being taken from the same points, was remarkable. We believe that the small height of the posterior fossa in the CMI patients might indicate bone hypoplasia and cause the posterior fossa organs to slope down.
Sekula et al. (3), Nishikawa et al. (8), and Hwang et al. (27) found the length of the superior and inferior cerebellar hemisphere to be higher in the CMI patients. However, only Hwang et al. (27) highlighted that the difference was statistically significant.
In our study, we found that the maximum height of the cerebellum was 59.13 ± 5.33 mm in the CMI group and 53.32 ± 5.37 mm in the controls. This difference between the CMI and control groups was statistically significant (P = 0.001).
Hwang et al. (27) found that the axial length of the cerebellar hemisphere (the most remote lateral distance) was 86.93 mm in the CMI group and 98.83 mm in the control group; they reported that the difference was not statistically significant.
In this study, we found the above parameter to be 104.65 ± 4.93 mm in the CMI group and 105.51 ± 5.75 mm in the control group. This difference between the patients and the controls was statistically insignificant (P = 0.249).
The results for this parameter showed similarities with the results of the study by Hwang et al. (27).
In our study, we found that the axial length of the cerebellar hemisphere on the midsagittal section was statistically greater in the CMI patients.
On the other hand, in the measurements they carried out on the transverse section, Hwang et al. (27) found that the axial length of the cerebellar hemisphere on the midsagittal section was statistically less in the CMI patients.
Unlike the other studies, we measured other parameters in this study such as maximum cranial height, occipital cord, cranial base length, and maximum cranial width in the CMI patients. We found the maximum cranial height to be 130.33 ± 6.17 mm in the CMI group and 132.94 ± 7.09 mm in the controls. This distance was statistically shorter in the CMI group (P = 0.004), which suggests that the total size of the cranium might be smaller in the CMI patients.
No statistical significance was found in the differences between the CMI and control groups in terms of the anteroposterior diameter of the cerebrum, maximum height of the cerebrum, and maximum cerebrum weight that we measured to ascertain whether the size of the cerebrum might affect CMI.
In conclusion, the size of the posterior cranial fossi was smaller and the size of the cerebellum was greater in the CMI patients. Thus, the risk of tonsillar herniation may be higher than the normal population in patients with a small posterior cranial fossa and a large cerebellum.