1. Background
Mitral regurgitation frequently accompanies dilated cardiomyopathy (DCM), which is associated with a poor prognosis (1). The increasing severity of mitral regurgitation, especially in moderate/severe patients remains an independent predictor of heart failure and sudden cardiac death (2, 3). Because DCM has multiple etiologies and morphological features of the disease, the detailed mechanism for coexistence of mitral regurgitation in DCM is complex and controversial. Several studies have shown that mitral regurgitation in DCM patients is caused by left ventricle (LV) and left atrium (LA) dysfunction and mitral valve complex abnormalities (4-6). However, whether the severity of mitral regurgitation in DCM patients correlates with the morphological and functional abnormalities of the left heart is uncertain.
With its high spatial and temporal resolution, magnetic resonance imaging (MRI) is regarded as the gold standard for the assessment of cardiac function and structure. Compared to 1.5 T MRI, 3.0 T MRI increases the signal-to-noise ratio (SNR) for the myocardium and blood, allowing accurate assessment of ventricular and atrial dysfunction, visualization and quantification of the morphology of the mitral valve (7, 8). These advantages can overcome the limitations of conventional echocardiography, such as the low spatial resolution and significant observer variability, which can affect the measurements’ reproducibility and credibility (9). The clinical LV function parameter data of DCM patients have been well documented by echocardiography (10); whereas, to our best knowledge, morphological and functional abnormalities on 3.0T MRI that may assist in prediction of mitral regurgitation severity are lacking.
2. Objectives
The aim of this study was to evaluate whether 3.0T MRI could estimate the correlation between mitral regurgitation and morphological, functional abnormalities of the left heart in DCM patients and whether this could assist in the prediction of the severity of mitral regurgitation.
3. Patients and Methods
3.1. Patient Selection
The study was approved by the institutional review board of our medical school. Written informed consent was obtained from all patients and controls.
From January 2014 to February 2016, we examined 47 consecutive DCM patients who underwent cardiac MRI at our medical center. The diagnosis of DCM was based on increased LV dimensions (left ventricular end-diastolic diameter ≥ 3.2 cm/m2) (11), the presence of a globally decreased contraction pattern (the contraction pattern is assessed using a grading system: hyperkinesia, increased wall motion; normokinesia, normal wall motion toward the center of the ventricle; hypokinesia, decreased wall motion; akinesia, absent wall motion. Hypokinesia and akinesia are decreased contraction patterns) (12) measured by echocardiography, and evidence of normal coronary arteries on coronary angiography or coronary computed tomography angiography. The exclusion criteria consisted of the presence of organic heart valvular disease or other combined cardiovascular diseases (n = 13), an incomplete MRI examination (n = 1), and non-interpretable MRI image quality (n = 2). Thus, 41 patients (mean age, 43 ± 18 years; range, 15 to 79 years) were enrolled in the study and underwent two-dimensional (2D) transthoracic echocardiography (TTE) and MRI. These patients included 29 men (mean age, 41 ± 16 years; range, 16 to 74 years) and 12 women (mean age, 47 ± 20 years; range, 15 to 79 years). No significant difference was found in age between the men and women (P = 0.40).
3.2. MRI
All patients were examined with a 3.0-T whole-body scanner (Trio Tim; Siemens medical solutions, Erlangen, Germany) with a maximum gradient strength of 50 mT/m and a maximum flew rate of 200 mT/m/ms. Electrocardiographic gating and breath holding in expiration were used when feasible. Long-axis two-, three-, and four-chamber images were acquired with a segmented echocardiography-gated Turbo FLASH cine sequence (slice thickness: 8 mm, repetition time: 37.66 ms, echo time: 1.2 ms, flip angle: 50°). Data acquisition was performed during end-inspiratory breath holding. For calculation of functional parameters, 8 - 12 serial short-axis slices from the base to the apex were acquired with an echocardiography-gated Turbo FLASH cine sequence (slice thickness: 10 mm, repetition time: 37.66 ms, echo time: 1.2 ms, flip angle: 50°, spacing between slices: 0 mm). Furthermore, the planimetry of the mitral annulus area was measured on the basal slice of the short-axis, which was demonstrated to be the mitral valve annulus.
3.3. Echocardiography
2D TTE and MRI were performed within 7 days with a multi-planar 1.5 - 3 MHz probe (IE33; Philips medical systems, Andover, Mass). With the patient in left lateral decubitus position, images were acquired in the parasternal, subcostal, and apical two-, three-, and four-chamber views for assessment of the degree of mitral regurgitation. The mitral regurgitation grading was classified with a three-point scale based on the ratio of absolute area of regurgitation jet to the size of the left atrium (mild, less than 20%; moderate, 20% - 40%; severe, greater than 40%) (13). Because the prognosis and clinical treatments are quite different with different mitral regurgitation grades, especially in moderate/severe mitral regurgitation patients (3, 14), we divided DCM patients into two subgroups: no/mild mitral regurgitation and moderate/severe mitral regurgitation.
3.4. Image Analysis
For MRI images, LV volumes and ejection fraction (EF) were measured with commercially available software (Argus; Siemens Medical Solutions) using serial short-axis slices. The endo- and epicardial borders were traced during the end-diastole and end-systole to obtain the volumetric parameters with the section summation method. For LA end-diastolic and end systolic volume (LAEDV, LAESV), both end-diastolic and end-systolic contours were traced in two-chamber and four-chamber views and calculated using biplane area-length method (15).
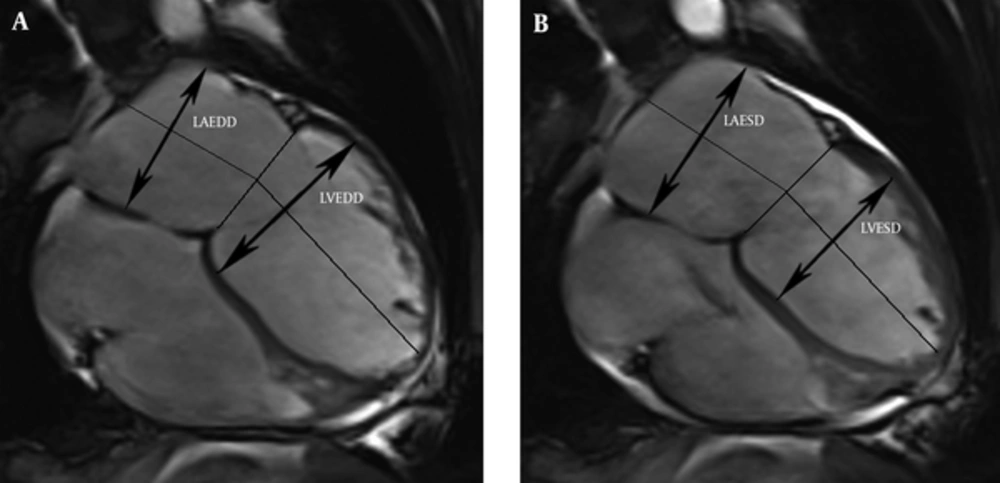
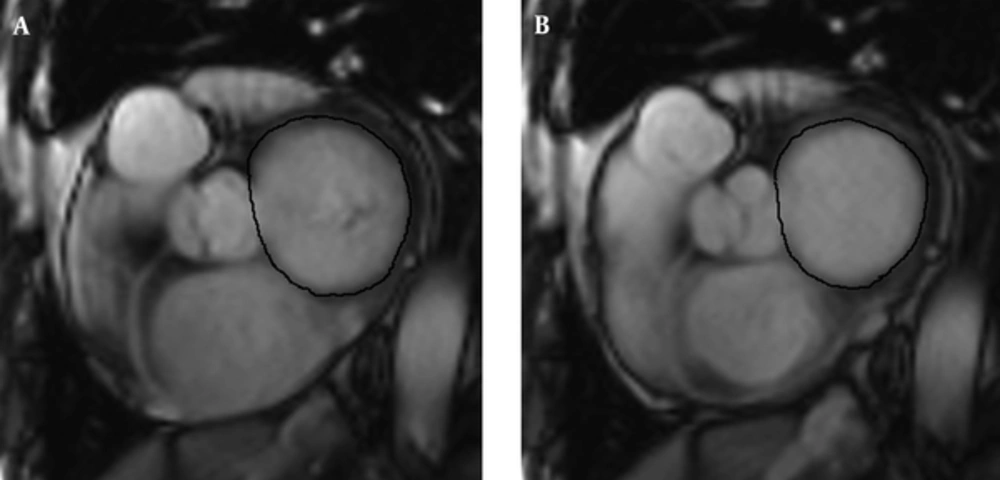
The LV and LA dimensions were determined on four-chamber views (Figure 1). The LV dimensions were measured as the maximal distance between the interventricular septum and lateral ventricular endocardium, which was perpendicular to the long axis in end-systole and end-diastole. The LA dimensions were defined as the maximal distance perpendicular to atrial length in end-systole and end-diastole, while the atrial length was measured as the distance between the midwall of the left atrium and the midpoint of the mitral annulus. The maximum and minimum mitral annulus areas (MAAmax and MAAmin) were planimetered at the mitral valve annulus slice (Figure 2). The volumes and dimensions of the LV, LA, MAAmax and MAAmin were indexed to body surface area (BSA).
Measurement of the left atrium and left ventricular dimensions from four-chamber views in the end-diastole (A) and end-systole (B). (LAEDD, left atrial end-diastolic dimension; LVEDD, left ventricular end-diastolic dimension; LAESD, left atrial end-systolic dimension; LVESD, left ventricular end-systolic dimension).
3.5. Variability Analysis of MRI Measurements
All of the MRI images were analyzed initially by one experienced radiologist who was blinded to the clinical data. To control intraobserver variability, the image analysis was repeated by the same investigator 1 month later. To control interobserver variability, a second experienced investigator independently reanalyzed the measurements without prior knowledge of the clinical data.
3.6. Statistical Analysis
Continuous and categorical variables were expressed as means ± SD and numbers (percentages), respectively. For all continuous categories, skewness and kurtosis were used to test for normality, and Levene's test was used to test for homogeneity of variance. Comparisons of two normally distributed variables were performed with Student’s t test; otherwise, Kruskal Wallis tests were used. The intraclass correlation coefficient (ICC) was used to calculate the interobserver and intraobserver variability. DCM patients were divided into four groups based on the presence and grading of mitral regurgitation. Correlation between mitral regurgitation grading and MRI parameters was assessed with Spearman’s rank correlation analysis. Receiver operating characteristic analysis was used to predict the severity of mitral regurgitation. All statistical analyses were performed using SPSS software (version 16.0 for windows; SPSS Inc., Chicago, Illinois, USA). A 2-tailed P value < 0.05 was considered statistically significant.
4. Results
4.1. Patient Characteristics
A total of 41 DCM patients were eligible for the study (Table 1). Echocardiographic examinations revealed 11 patients (27%) without mitral regurgitation and 12 patients (29%) with mild, 11 (27%) with moderate, and 7 (17%) with severe mitral regurgitation. According to the New York heart association (NYHA) classification, 5 (12%), 8 (20%), 16 (39%) and 12 (29%) patients had a class of I, II, III, and IV, respectively. No differences in mitral, LA and LV parameters were evident among DCM patients with respect to the NYHA class.
| Parameter | DCM Patients (n = 41) | Healthy Subjects (n = 26) | P |
|---|---|---|---|
| Age, y | 43 ± 18 | 39 ± 14 | 0.39 |
| Men, No. (%) | 29 (70.7) | 14(53.9) | 0.21 |
| BSA, m2 | 1.6 ± 0.2 | 1.6 ± 0.1 | 0.98 |
| BMI, kg/m2 | 21.9 ± 4.6 | 22.7 ± 3.6 | 0.62 |
| Systolic pressure | 116.9±17.5 | 122±5.9 | 0.10 |
| Diastolic pressure | 74.8±11.9 | 79±4.6 | 0.19 |
| NYHA, I, II, III, IV | 5/8/16/12 | - | - |
Baseline Characteristics of DCM Patients and Normal Individualsa
4.2. Morphological and Functional Abnormalities of the Left Heart and Mitral Valve
Data for the parameters of the mitral valve, LA and LV are summarized in Table 2. The EF values of DCM patients were significantly lower than those of healthy subjects (23.6 ± 11.7% vs. 61.3 ± 6.6%, P < 0.001). The indexed end-diastolic volume (EDV) and end-systolic volume (ESV), the indexed end-diastolic dimension (EDD) and end-systolic dimension (ESD) of the LV and LA were significantly higher in patients than in control subjects (all P < 0.005). In DCM patients, both the indexed MAAmin (9.9 ± 4.0 cm2/m2 vs. 5.9 ± 1.2 cm2/m2, P = 0.001) and indexed MAAmax (12.9 ± 3.8 cm2/m2 vs. 9.5 ± 1.1 cm2/m2, P = 0.002) were significantly larger than in healthy subjects.
| Parameters | Healthy Subjects | DCM Patients | P Value |
|---|---|---|---|
| LV and LA Parameter | |||
| Indexed LVEDV, mL/m2 | 71.5 ± 11.0 | 163.8 ± 73.8 | < 0.001 |
| Indexed LVESV, mL/m2 | 27.6 ± 5.7 | 131.8 ± 74.3 | < 0.001 |
| LVEF (%) | 61.3 ± 6.6 | 23.6 ± 11.7 | < 0.001 |
| Indexed LAEDV, mL/m2 | 45.2 ± 14.1 | 84.6 ± 37.3 | 0.001 |
| Indexed LA ESV, mL/m2 | 19.7 ± 5.7 | 59.2 ± 36.2 | 0.001 |
| Indexed LVEDD, cm/m2 | 2.8 ± 0.4 | 4.2 ± 0.8 | < 0.001 |
| Indexed LVESD, cm/m2 | 1.7 ±0.2 | 3.7 ± 1.9 | < 0.001 |
| Indexed LAEDD, cm/m2 | 2.4 ± 0.4 | 4.1 ± 0.8 | < 0.001 |
| Indexed LAESD, cm/m2 | 1.7 ± 0.3 | 3.3 ± 0.8 | < 0.001 |
| Mitral valve parameter, cm2/m2 | |||
| Indexed MAAmin | 5.9 ± 1.2 | 9.9 ± 4.0 | 0.001 |
| Indexed MAAmax | 9.5 ± 1.1 | 12.9 ± 3.8 | 0.002 |
Comparison of MRI Parameters Between DCM Patients and Healthy Subjectsa
4.3. Correlation Between Mitral Regurgitation Grading and Morphological, Functional Abnormalities
Within the DCM group, the Spearman correlation test indicated a good correlation between grading of mitral regurgitation and the function. Morphological parameters are listed in Table 3. They show a good correlation (r = 0.43 to 0.61; all P < 0.05) but not for EF values (r = -0.38). The indexed LVESD, LAESD and MAAmin tended to increase with increasing grades of mitral regurgitation (r = 0.58, 0.61, and 0.56, respectively, all P < 0.001). Moreover, a significant correlation was found between indexed LVESD and LAEDD in patients with DCM (r = 0.92; P < 0.001). A moderate but significant correlation was evident between indexed MAAmin and LAEDD (r = 0.47, P = 0.004), and indexed MAAmin and LVESD (r = 0.45, P = 0.005).
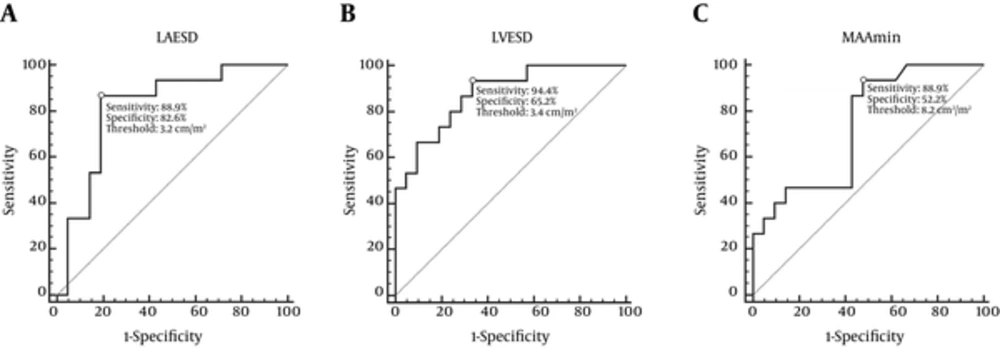
According to the above-mentioned criteria, 23 patients (56%) had no/mild mitral regurgitation, and 18 patients (44%) had moderate/severe mitral regurgitation. Patients with moderate/severe mitral regurgitation showed lower BSA and BMI values than that of no/mild mitral regurgitation patients (P < 0.05). The MRI parameters of two subgroups are listed and compared in Table 3. All morphological and functional parameters of LV, LA, and mitral valve in DCM patients with moderate/severe mitral regurgitation were greater than that of no/mild mitral regurgitation patients (all P < 0.05), except for EF values (19.3 ± 7.5% vs. 26.6 ± 13.5%, P < 0.05). As shown by diagnostic statistics in Table 4, the indexed LVESD, LAESD and MAAmin might be used to distinguish no/mild mitral regurgitation patients from moderate/severe mitral regurgitation patients with high sensitivity and specificity (areas under receiver operating characteristic curve [AUC] = 0.876, 0.816, and 0.773, respectively, all P < 0.05) (Figure 3). When a single parameter was used, a relatively higher AUC (0.816), sensitivity (88.9%) and specificity (82.6%) could be achieved with a cutoff of 3.2 cm/m2 for indexed LAESD. The highest negative predictive value (93.8%) and sensitivity (94.4%) were obtained when the indexed LVESD was greater than 3.4 cm/m2. When a combination of the indexed LAESD, LVESD and MAAmin was used, the specificity was increased to 91.3%.
Receiver operating characteristic plot for predicting moderate/severe mitral regurgitation. A, Sensitivity and specificity of LA enlargement (LAESD> 3.2 cm/m2) for diagnosis of moderate/severe mitral regurgitation were 88.9% and 82.6%, respectively. B, Sensitivity and specificity of LV enlargement (LVESD> 3.4 cm/m2) for diagnosis of moderate/severe mitral regurgitation were 94.4% and 65.2%, respectively. C, Sensitivity and specificity of MAAmin enlargement (MAAmin> 8.2 cm2/m2) for diagnosis of moderate/severe mitral regurgitation were 77.8% and 91.3%, respectively. (LAESD, left atrial end-systolic dimension; LVESD, left ventricular end-systolic dimension; MAAmin, minimum mitral annulus area)
| Parameters | No/Mild | Moderate/Severe | P | Spearman Correlation r (p) |
|---|---|---|---|---|
| Patients (n) | 23 | 18 | ||
| LV and LA parameter | ||||
| Indexed LVEDV, mL/m2 | 134.0 ± 35.8 | 205.5 ± 92.6 | 0.003 | 0.44 (0.007) |
| Indexed LVESV, mL/m2 | 101.2 ± 40.7 | 174.6 ± 89.8 | 0.002 | 0.43 (0.01) |
| LV EF (%) | 26.6 ± 13.5 | 19.3 ± 7.5 | 0.06 | -0.38 (0.022) |
| Indexed LAEDV, mL/m2 | 73.7 ± 34.6 | 100.0 ± 36.8 | 0.04 | 0.52 (0.001) |
| Indexed LAESV, mL/m2 | 48.8 ± 35.3 | 73.7 ± 33.2 | 0.04 | 0.52 (0.001) |
| Indexed LV EDD, cm/m2 | 3.8 ± 0.6 | 4.7 ± 0.9 | 0.003 | 0.44 (0.006) |
| Indexed LVESD, cm/m2 | 3.2 ± 0.7 | 4.3 ± 0.8 | 0.001 | 0.58 (0.000) |
| Indexed LAEDD, cm/m2 | 3.8 ± 0.6 | 4.7± 0.9 | 0.001 | 0.45 (0.006) |
| Indexed LAESD, cm/m2 | 3.0 ± 0.8 | 3.8 ± 0.6 | 0.001 | 0.61 (0.000) |
| Mitral Valve Parameter,cm2/m2 | ||||
| Indexed MAAmin | 8.3 ± 3.0 | 12.1 ± 4.3 | 0.003 | 0.56 (0.000) |
| Indexed MAAmax | 11.6 ± 2.9 | 14.8 ± 4.1 | 0.009 | 0.44 (0.008) |
MRI Parameters in DCM Patients with Different Severity of Mitral Regurgitationa
| Morphologic Parameter | Sensitivity (%) | Specificity (%) | Accuracy (%) | Positive Predictive Value (%) | Negative Predictive Value (%) |
|---|---|---|---|---|---|
| LAESD > 3.2, cm/m2 (1) | 88.9 (16/18) | 82.6 (19/23) | 85.4 (35/41) | 80 (16/20) | 90.5 (19/21) |
| LVESD > 3.4, cm/m2 (2) | 94.4 (17/18) | 65.2 (15/23) | 78.0 (32/41) | 68.0 (17/25) | 93.8 (15/16) |
| MAAmin > 8.2, cm2/m2 (3) | 88.9 (16/18) | 52.2 (12/23) | 68.3 (28/41) | 59.3 (16/27) | 85.7 (12/14) |
| (1) + (2) | 88.9 (16/18) | 87.0 (20/23) | 87.8 (36/41) | 84.2 (16/19) | 91.0 (20/22) |
| (1) + (2) + (3) | 77.8 (14/18) | 91.3 (21/23) | 73.2 (35/41) | 87.5 (14/16) | 84 (21/25) |
Prediction of Moderate/Severe Mitral Regurgitation in DCM Patients with Morphologic Parametersa
4.4. Variability of MRI Measurement
The interobserver and intraobserver variability showed an excellent agreement among MRI measurements as follows: the ICC of intraobserver variabilities for measurement of indexed LVEDV, LVESV, LAEDD, LAESD, and MAAmin were 0.97, 0.95, 0.97, 0.95, and 0.92, respectively; the ICC of interobserver variabilities for measurement of indexed LVEDV, LVESV, LAEDD, LAESD, and MAAmin were 0.95, 0.96, 0.98, 0.91, and 0.89, respectively.
5. Discussion
In this study, we found that mitral regurgitation frequently coexists in DCM patients. With increasing severity of mitral regurgitation, the indexed LVESD, LAESD and MAAmin tended to increase accordingly and might help to distinguish no/mild mitral regurgitation patients from moderate/severe mitral regurgitation patients.
DCM is a disease with multiple etiologies and morphological features. The chief characteristic of DCM is dilation of all chambers and impaired function of one or both ventricles, especially the left ventricle. Additionally, mild focal scarring of the mitral valve and dilation of the annulus are frequently observed (1, 16). In our study, echocardiographic examinations revealed that the proportion of DCM patients with mitral regurgitation was 73%. The proportion of moderate/severe mitral regurgitation patients in our study was 44% (18/41), which was much higher than in Anwar’s report (20%, 4/20). (17). The reason for this difference could be different demographics of the patients.
As the most crucial systolic function parameter, EF is of great clinical importance during diagnosis, therapeutic assessment and prognostic prediction of cardiovascular disease. In our study, we found that EF in DCM patients with moderate/severe mitral regurgitation significantly decreased compared to that of no/mild mitral regurgitation patients, which was consistent with previous studies (18, 19). The impaired LV systolic function always occurs in concomitance with mitral regurgitation in DCM. The decreased EF in DCM frequently accompanies ventricular enlargement, resulting in deformation of the mitral complex, especially the mitral annulus. LV volume overload with increased preload secondary to mitral regurgitation hemodynamically affect EF. DCM patients with moderate/severe mitral regurgitation showed a larger LV volume and dimension, increasing the LV preload more prominently than no/mild patients, which in turn significantly decreased EF.
The normal closure of the mitral valve requires good coordination of the LA, LV, mitral annulus, mitral leaflets and the subvalvular apparatus (20). In DCM patients, chamber dilatation and alteration in the geometric relationship of the mitral valve apparatus may lead to mitral regurgitation, particularly MAA dilatation. According to previous studies (2, 3, 21), the presence of mitral regurgitation is associated with a poor outcome, and a moderate/severe degree of mitral regurgitation remains an independent predictor for sudden cardiac death, heart failure, and mortality in DCM patients. Surgical correction for severe mitral regurgitation can improve patients’ symptoms and quality of life, reduce hospital mortality, and reveal reverse LV remodeling (14, 22). Thus, morphological and function assessment of LA, LV, and MAA plays a key role in the therapeutic evaluation of DCM patients with significant mitral regurgitation. Precise measurement of MAA can help clinicians to choose appropriate prosthetic mitral valve rings and percutaneous annuloplasty devices (23, 24).
Although echocardiography is available and widely used for the diagnosis of DCM and LV function assessment, the low spatial resolution and operator-dependent characteristics hamper the accuracy quantification of parameters, especially volumetric parameters (25). MRI has been proven of clinical value for assessment of cardiac function and structure (7, 8, 26). The unique challenges posed by cardiac imaging such as physiological motion and position of the heart can decrease image quality, especially SNR in low filed MRI (8, 27). Three tesla MRI overcomes the limitation of 1.5 T MRI and extends the capabilities of cardiac MRI. As the magnetic field strength increases, the bulk magnetization increases as well, resulting in a linear increasing of SNR (8). The improvement in SNR supports excellent delineation of the blood-myocardium interface and hence facilitates precise quantification of cardiac function. In addition, 3.0 T MRI allows reproducible and accurate measurement of detailed anatomy of the left heart such as the left ventricular, LA, and mitral valve (15, 17). These advancements of MRI may be beneficial for assessing functional and morphological features of the left heart and predicting the severity of coexistence of mitral regurgitation in DCM patients.
Previous studies have debated whether mitral regurgitation is caused by dysfunction of the ventricle, mitral valve or atrium (4-6, 28). In our study, we have found that all morphological and functional parameters of the LV, LA, and mitral valve in DCM patients with moderate/severe mitral regurgitation were greater than that of no/mild mitral regurgitation patients, and the indexed LVESD, LAESD and MAAmin tended to increase with increasing grades of mitral regurgitation. Furthermore, there was a significant correlation between indexed LVESD and LAEDD in patients with DCM. This suggested that DCM patients with a decreased LVEF had an increased risk proportional to increase in LA size due to the increasing after load. Similarly, previous reports have found that the main determinant of LA volume is ventricular diastolic function. Diastolic dysfunction should be regarded as an important contributing factor to the development of mitral regurgitation in DCM patients, likely by causing enlargement of LA and mitral annulus (4). Therefore, our results further confirmed that a combination of dilation and impaired function of the left heart with a larger MAA contribute to the occurrence and development of mitral regurgitation in DCM patients.
Recognition of the presence and severity of mitral regurgitation, especially a moderate/severe degree of mitral regurgitation, can be of great value for risk stratification, treatment decisions and prognosis prediction (2, 3, 21). Our studies have revealed that the indexed LVESD, LAESD, and MAAmin are positively correlated with increasing grades of mitral regurgitation grading, and in particular, the indexed LVESD is sufficiently sensitive to detect moderate/severe mitral regurgitation. A combination of indexed LAESD and LVESD produced relatively higher sensitivity, specificity, positive predictive value, and negative predictive value. Further combination of indexed MAAmin with LAESD and LVESD produced the highest specificity (91.3%) for predicting mitral regurgitation severity. Moreover, inter- and intraobserver variability analysis indicated that 3.0 T MRI appears to be an accurate and reliable modality for measuring these morphological parameters.
We acknowledge the following limitations of our study. A unicenter design allows for a consistent scanning protocol and excellent reproducibility in MRI but may also cause selection bias. The diagnostic value of the LV, LA and mitral valve in DCM patients with mitral regurgitation needs to be proven in larger cohort studies. Furthermore, the prognostic role of dysfunction and morphological measurements on cardiac MRI for survival in DCM needs further clinical investigation.
In conclusion, dysfunction and dilation of LV, LA, and mitral annulus in DCM patients with mitral regurgitation can be reliably and accurately determined by MRI. The indexed LVESD, LAESD, and MAAmin tended to increase simultaneously with increasing grades of mitral regurgitation, which can assist in the prediction of mitral regurgitation severity with high sensitivity and specificity.