1. Background
Percutaneous vertebroplasty (PV) is a well-established treatment for stabilization of vertebral bodies in both vertebral fractures and vertebral metastasis (1). Osteoporosis is the most common cause of fractures occurring at different anatomical sites, and one of the most common is fracture of vertebral bodies (2, 3). Moreover, the most common site of bone metastases are the vertebrae, which is likely due to their high vascularization (4), and this is of particular clinical importance in the breast, lung, kidney, and prostate cancers (5-8).
Because of the prevalence of these diseases, vertebral body metastases (VBM) and vertebral fractures represent the most common etiologies of chronic pain (9). The utility and effectiveness of PV is most often evaluated on the basis of short- and long-term pain relief, and the quality of life (10).
Many different scores are used to evaluate patients’ pain, vertebral disease related pain, and quality of life. One of the most common available score systems is the visual analogue scale, which is simple and easy to use, even if not designed specifically for back pain. A number of disease-specific questionnaires have been developed to assess low-back pain (11-14).
2. Objectives
The purpose of this study was to assess the efficacy of pain control at a single center in a population of patients with osteoporotic fractures or vertebral metastasis who received vertebroplasty.
3. Patients and Methods
3.1. Patients
We retrospectively evaluated all patients who underwent vertebroplasty at our institution between January 2008 and March 2016. Institutional review board approval was obtained for the study, and a written informed consent was gathered from all patients.
We included patients who underwent vertebroplasty (VP) for 1) painful primary and secondary osteoporotic vertebral fractures refractory to conservative medical therapy and 2) painful vertebrae with extensive osteolysis or invasion secondary to a benign or malignant tumor (e.g., myeloma, hemangioma, or metastasis) (15).
The exclusion criteria were (1) involvement in more than two vertebral bodies, (2) cervical vertebrae involvement, (3) patients younger than 18 years or older than 85 years, and (4) performance of other procedures in addition to VP in the same session (e.g., thermoablations or nucleoplasty).
Absolute contraindications were (1) asymptomatic spine fractures, (2) presence of local or systemic infection, (3) retropulsed bone fragment resulting in myelopathy, (4) spinal canal compression due to tumor development resulting in myelopathy, (5) uncorrectable coagulopathy, and (6) allergy to the materials used in the vertebroplasty procedure (15).
Data on 231 patients who met the inclusion criteria were collected for this study. Seventy-one patients were excluded because they met the exclusion criteria: 17 had VP performed on three vertebrae, one had VP performed on four vertebrae, five patients underwent cervical VP, three were over 90 years old, and 43 underwent previous thermoablation.
Vertebral body fractures or osteolysis were detected using plain films or cross-sectional imaging, computed tomography (CT), or magnetic resonance imaging (MRI).
3.2. Pre-procedure Imaging and Procedure Guidance
All patients underwent a pre-procedure MRI or CT scan of the spine, including at least two vertebral bodies, one above and one below the suspected level of the fracture. CT reconstruction scans provided 1 mm axial, coronal and sagittal multiplanar reconstructions (MPRs). A pre-procedure CT or MRI was used to estimate the extent of vertebral compression and bone impairment, to detect retropulsed bone fragments or posterior wall interruptions, and to plan access to the vertebral body.
3.3. Procedure and Follow-up
Patient admission at the hospital was performed the day prior to the procedure. Patients’ pain and disabilities were assessed using two scoring systems. Pain was estimated with the visual analogue scale (VAS), which provides a scale from 0 (no pain) to 10 (worst pain imagined). Pain related disability was measured using the modified Roland-Morris questionnaire (RMQ), Italian version (14), which provides 24 assessments related to back pain and daily activities (scale from 0 to 24 points; the latter represents the worst score).
All patients received prophylactic antibiotic therapy intravenously before the procedure (750 mg of cefuroxime). All procedures were performed using CT guidance under conscious sedation (general anesthesia was performed for patients who did not tolerate sedation). The operator decided on a personal preference to use fluoroscopic guidance with a portable C-arm in the CT suite. All procedures were conducted with the patient in the prone position. We used a posterior percutaneous approach at all levels (providing an extrapedicular or transpedicular approach above T10 and below T10, respectively).
In all cases, we used a 10-gauge vertebroplasty needle (Optimed, Ettlingen, Germany). A unipedicular or bipedicular access was performed on the basis of the operator’s preference. A slow injection of 2-6 ml of polymethylmethacrylate (PMMA) was given (Osteopal 40 and Osteopal V, Biomet Deutschland GmbH, Berlin, Germany). The injection was gently and slowly performed using an Optimed Gangi Cemento-Re Gun (Optimed, Ettlingen, Germany) under intermittent CT fluoroscopy or fluoroscopy with gradual withdrawing of the needle. A CT scan was performed immediately after needle removal. Patients were kept on strict bed rest for 2 hours and discharged the same day of the procedure or the day after. VAS and Roland-Morris questionnaire (RMQ) values were calculated at one month and 6 months after the treatment on an outpatient basis or by phone.
3.4. Statistics
Descriptive statistics were calculated for all variables evaluated, reporting raw numbers, frequencies and averages. Student’s t test was used to test for significant differences between continuous variables. A chi-squared test was used for discrete variables. Statistical analyses were performed using MedCalc Statistical Software version 14.8.1 (MedCalc Software bvba, Ostend, Belgium).
4. Results
A total of 163 patients were included in this study. The mean age was 71.5 years (SD 10.73; min 42, max 89). Fifty-three patients were male and 107 female. Only thirty patients had painful vertebrae osteolysis or invasion secondary to a benign or malignant tumor. Forty-three patients underwent two VPs in the same session. A total of 203 VPs were performed: 1 on D5, 2 on D6, 5 on D7, 6 on D8, 6 on D9, 11 on D18, 18 on D11, 29 on D12, 38 on L1, 27 on L2, 23 on L3, 25 on L4, and 12 on L5. Patients with osteoporotic fractures had a higher mean age (P = 0.003). The distribution of treated vertebrae was different considering osteoporotic or secondary to tumor fractures (P = 0.04). No difference was found between patients who underwent one or two vertebrae treatments in the same session (P = 0.06) or regarding sex (P = 0.27) between the two groups.
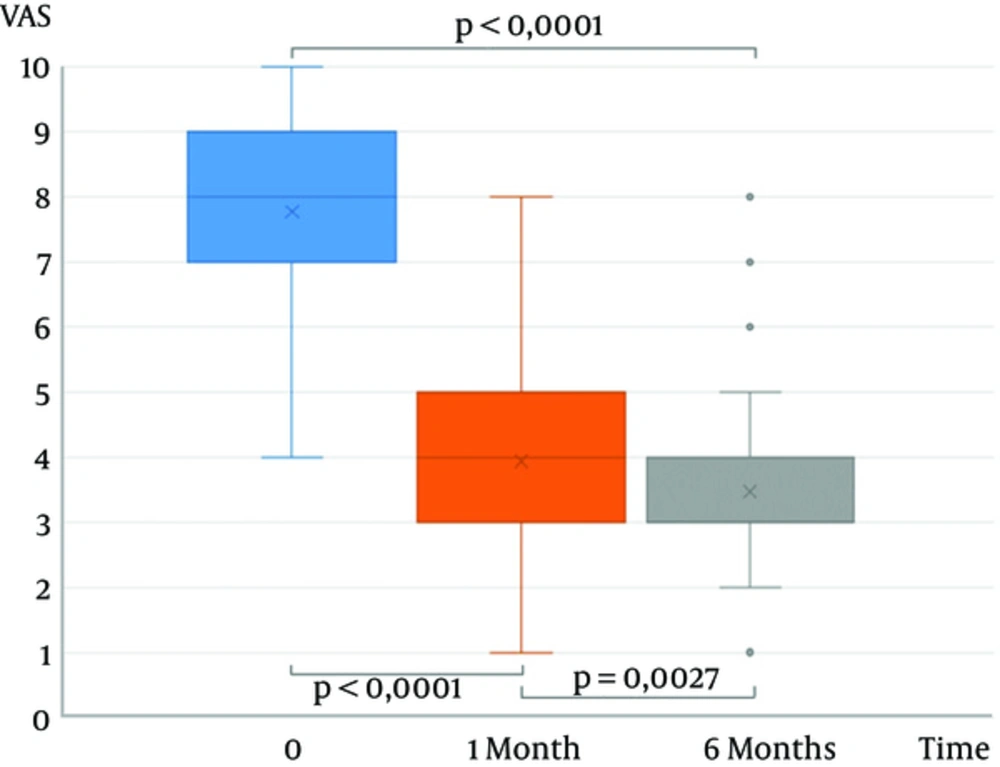
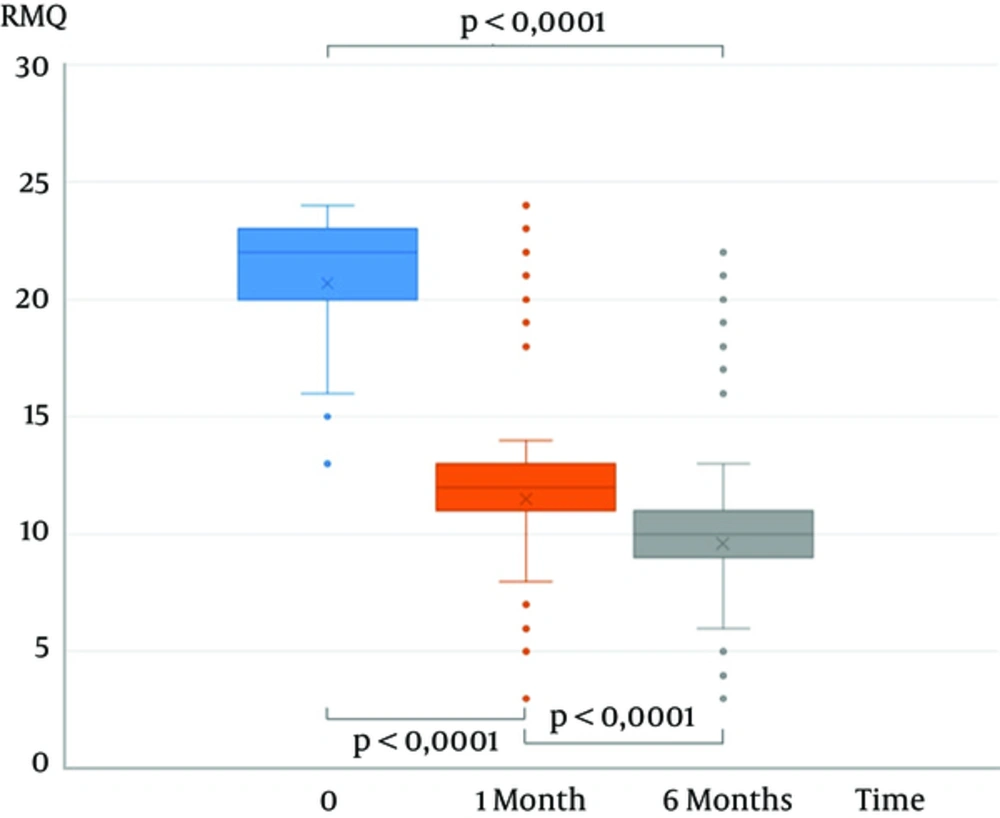
The VAS mean score was 7.8 (SD 1.2), 3.9 (SD 2.4), and 3.5 (SD 1.3) before the procedure and at 1 month and 6 months follow-up, respectively, showing a significant decrease (P < 0.0001) (Figure 1). The RMQ mean score was 20.7 (SD 3.2), 11.5 (SD 3.5), and 9.6 (SD 3.3) before the procedure and at 1 month and 6 months follow-up, respectively, showing a significant decrease (P < 0.0001) (Figure 2).
There was no difference in baseline VAS and RMQ scores, as well as at 1 month and 6 months follow-up, between patients with painful vertebrae secondary to benign or malignant tumors or osteoporotic vertebral compression fractures.
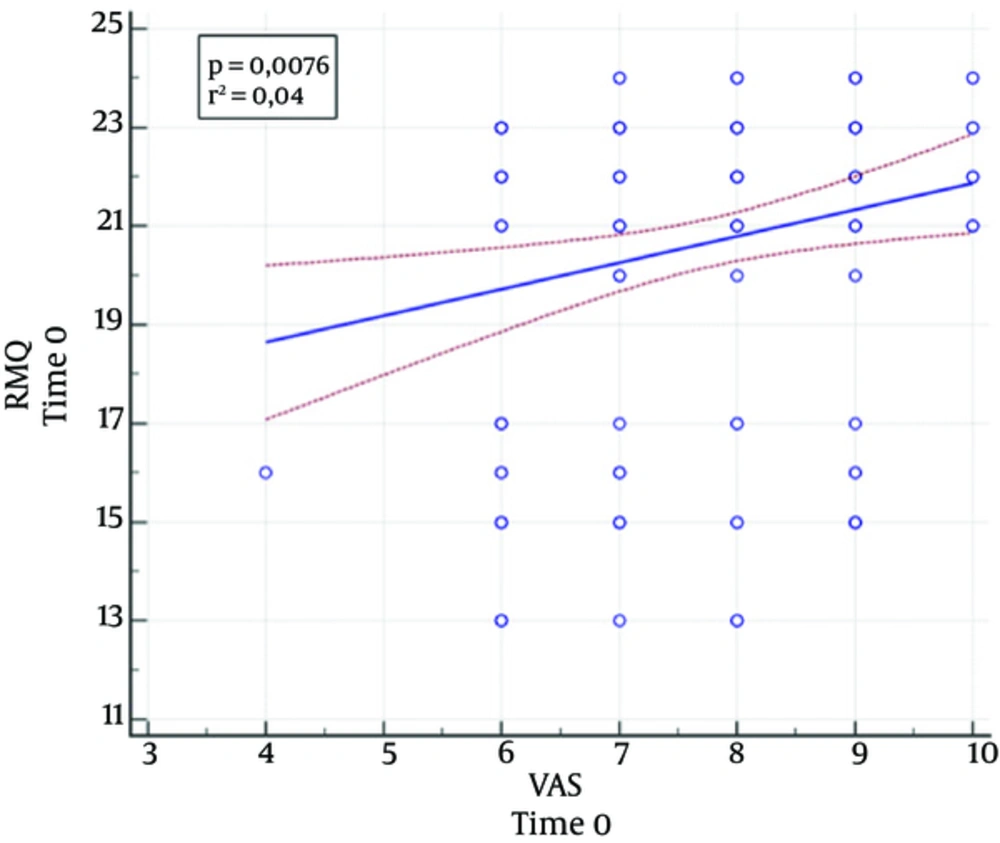
A significant correlation was noticed between the VAS scores and the RMQ scores at baseline (P = 0.008; r = 0.21) (Figure 3), but this correlation was not more evident at the 1 month (P = 0.62) and 6 months (P = 0.63) follow-up.
5. Discussion
This retrospective study evaluated the efficacy of VP for treatment of vertebral fractures due to osteoporosis or tumor infiltration showing a clinically significant pain relief and an improved quality of life.
To date, there have been many reports on VP for spinal fracture treatment associated with both tumor invasion and osteoporosis. Most of these reports demonstrated the efficacy of VP in reducing pain, aiding mobility and responding to physiotherapy, as well as improving the quality of life (16, 17).
Nevertheless, the level of evidence reached by this study is still not well established because some randomized clinical trials (RCTs) on the topic of percutaneous vertebral augmentation showed a trend of improvement in the short-term outcome compared with nonsurgical management and a placebo-simulated procedure (18, 19).
Yang et al. in their prospective RCT concerning patients older than 70 years demonstrated that early VP allows faster and better pain relief and improved functional outcomes, which lasted for 1 year and was accompanied by fewer complications than conservative treatment (20). In contrast, Macias-Hernandez et al. concluded that percutaneous VP had no advantages over conservative treatment for pain and function in a group of women ≥ 60 years of age with osteoporosis (21). Moreover, Clarençonet et al. recently suggested that VP remains a safe and effective technique for pain relief, independent of the underlying disease in patients ≥ 80 years of age (22).
In our series, VP seemed to be effective in the treatment of osteoporotic fractures with a significant improvement in pain and quality of life. Even in the case of neoplastic invasion, VP showed significant efficacy. The relief of pain was shown to be prompt, with good results already at 4 weeks after the procedure, with a slight further improvement at 6 months.
In cases of neoplastic invasion, many authors have proposed to precede the VP with thermal-ablation to have better control of the disease. These approaches showed promising results and appeared to be safe and effective in the treatment of painful neoplastic lesions (23). Nevertheless, the best effectiveness of this technique needs to be clearly demonstrated (24).
The present study validated the RMQ in both osteoporotic and neoplastic fractures, showing a strong correlation with VAS scores (14) at the baseline, while the absence of a correlation during the follow-up was probably due to the small gap between values since there was significant improvement from the baseline.
This study was limited by many factors. The retrospective nature of our study did not allow us to include an optimal control group, particularly comparing VP to medical treatment. The population was enrolled from a single center within a long time with the consequent possibility of an inhomogeneous approach. There is increasing evidence that optimizing patient outcomes results in improved quality of life and that pain management is an essential part of comprehensive management of oncologic and elderly patients.
Our study suggested that VP is a safe technique showing similar clinical effectiveness in terms of pain relief and quality of life independently of vertebral fracture etiology; however, these results need to be confirmed by prospective randomized controlled studies.