1. Introduction
Percutaneous transhepatic cholangiography (PTC) is a minimally invasive radiological technique used for the drainage of the obstructed biliary tree, primarily due to choledocholithiasis or tumors. In the absence of a stone or tumor, a duodenal diverticulum may rarely cause obstructive jaundice, a condition known as Lemmel syndrome (1). Herein, a case of obstructive jaundice caused by a bezoar in a duodenal diverticulum is described, along with its management using PTC.
2. Case Presentation
A sixty-five-year-old female patient was admitted to the emergency room with nausea, vomiting, and intermittent abdominal pain. She had no other known diseases or history of surgery. Her physical examination was unremarkable except for diffuse mild tenderness in the abdomen. At admission, her complete blood count was within normal limits except for an elevated leukocyte count (WBC: 11,770 µL) with neutrophilic predominance. Liver function enzymes were elevated (AST: 126 U/L, ALT: 316 U/L, ALP: 185 U/L, GGT: 305 U/L). The total bilirubin level was 6.8 mg/dL, and the direct bilirubin level was 5.7 mg/dL. Amylase and lipase levels were also slightly elevated at 452 U/L and 415 U/L, respectively.
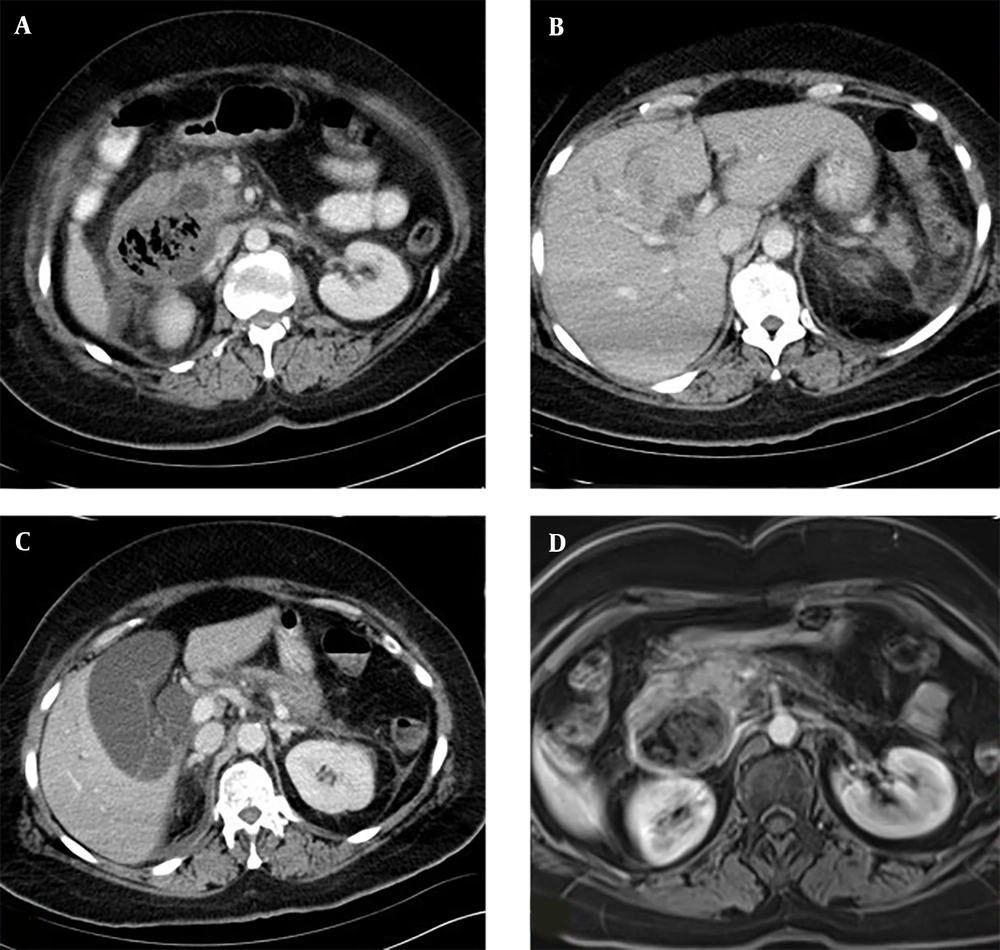
Abdominal ultrasonography (US) revealed a dilated biliary tree associated with a 5 cm diameter heterogeneous mass with gas echoes adjacent to the ampulla, raising suspicion of a tumor, an abscess, or a diverticulum. The patient was referred for a computed tomography (CT) scan and subsequent magnetic resonance imaging (MRI). The CT scan showed a duodenal diverticulum abutting the ampulla, filled with a large non-enhancing heterogeneous bezoar. Investigation into the etiology of the bezoar revealed that the patient did not have a psychiatric disease nor a habit of eating hair. It was considered that fibrous foods and fruits might have caused an advanced phytobezoar due to their indigestible components. MRI showed dilatation of both the biliary tree and the pancreatic duct. Multiple millimetric biliary stones were present in the gallbladder; however, no stones were detected along the common bile duct (CBD) or at the ampulla. The biliary dilatation was considered to be secondary to the compression or obstruction by the bezoar (Figure 1).
The patient underwent endoscopic retrograde cholangiopancreatography (ERCP), which confirmed the diagnosis of a diverticulum. The papilla was located in the diverticulum, but despite various attempts and maneuvers, it could not be cannulated. The bezoar was found to be extremely hard and could not be fragmented. Consequently, the patient was referred for PTC drainage.
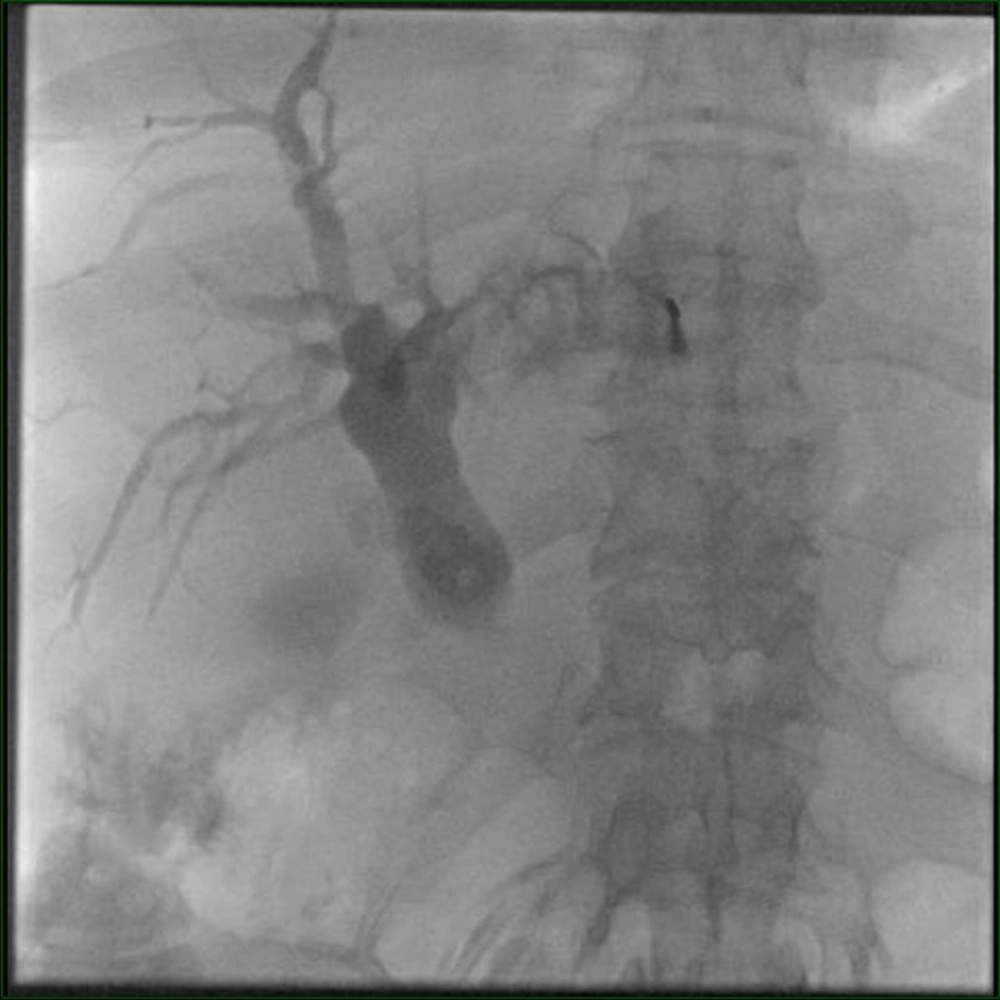
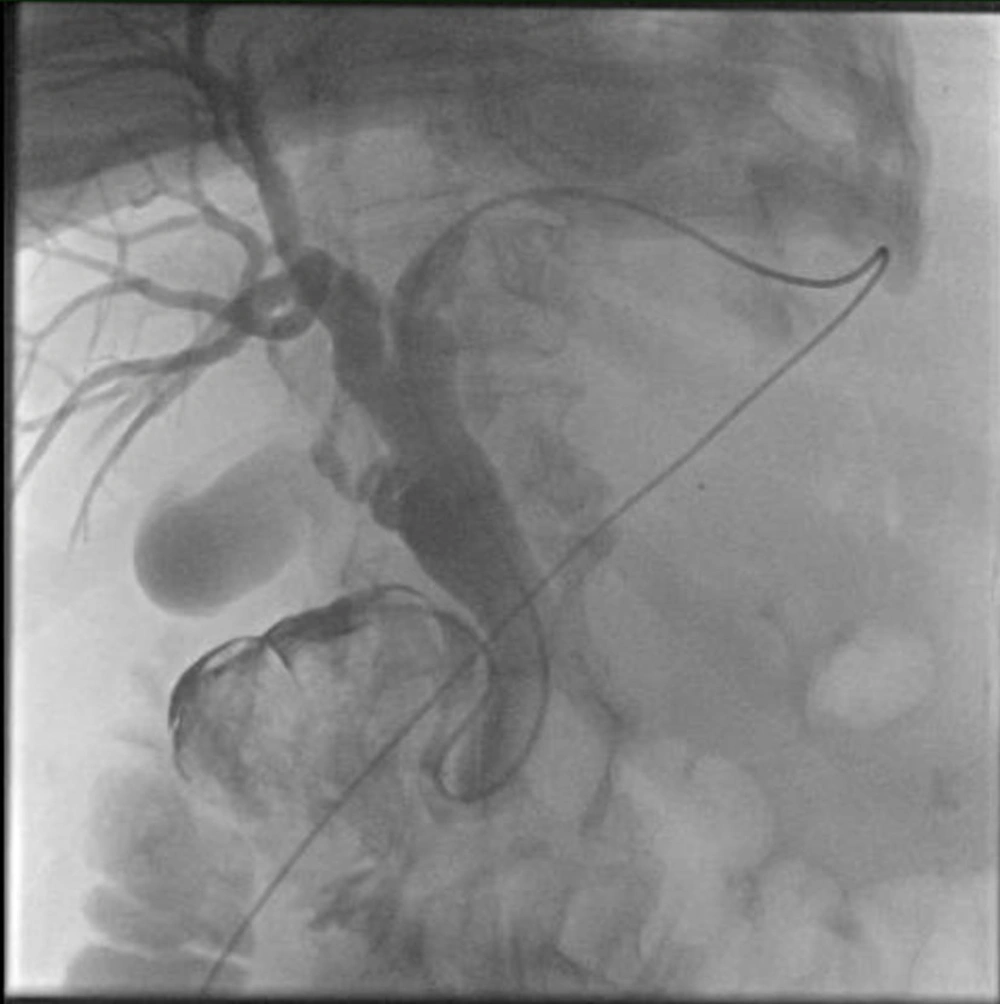
Under ultrasound guidance, the left intrahepatic biliary canal was punctured with a 22G needle, and a contrast agent was injected. Cholangiography demonstrated bilobar biliary dilatation extending to the ampulla. However, contrast agent passage to the duodenum was not observed (Figure 2). Through catheter and wire manipulations, a duodenal diverticulum was reached. A second injection showed contrast agent scattered around the bezoar, but no further distal passage was seen. Despite various maneuvers, the catheter could not be advanced to the jejunum. Therefore, an 8F external drainage catheter was placed in the common bile duct (Figure 3).
Under broad-spectrum antibiotics, liver enzymes and bilirubin levels dropped significantly after PTC drainage. As the patient's general condition improved, she was referred for surgery due to the large size of the diverticulum and the extremely hard bezoar. A diverticulectomy was performed, and the patient was discharged uneventfully.
3. Discussion
The majority of gastric bezoars are found in adolescents and young women with a history of pica, predominantly associated with psychiatric disorders. In contrast, most gastric bezoars in adults are linked to gastroparesis, anatomical abnormalities, and previous gastric surgeries that reduce gastric motility, ultimately resulting in delayed stomach emptying (2). Our patient had no history of pica or psychiatric disorders but did have an anatomical abnormality (diverticulum) in the duodenum. The prolongation of duodenal emptying due to the diverticulum may have contributed to the development of the bezoar.
Jaundice is the most common presentation in biliary obstruction cases, with the most frequent causes being choledocholithiasis and tumors. Duodenal diverticula, which are far more commonly acquired than congenital, may appear as cystic lesions near the pancreatic head if not filled with oral contrast material or ingested air. They are most often located along the medial wall of the second to third portions of the duodenum, usually within 2.0 cm of the ampulla of Vater. The presence of gas or air-fluid levels is a very helpful clue for the correct diagnosis (3). Although mostly asymptomatic, patients may occasionally present with pancreatitis or jaundice (4). Duodenal diverticula can make papillary cannulation difficult for gastroenterologists during ERCP, particularly if the ampulla drains into the diverticulum (3).
Knowing the findings of bezoars in imaging modalities is a critical step in patient management. Sonographic diagnosis of a bezoar is established by detecting an intraluminal mass with a hyperechoic arc-like surface and a marked acoustic shadow. In CT imaging, the diagnosis of a bezoar is based on identifying a low-density intraluminal mass containing air bubbles and exhibiting a characteristic mottled appearance (5, 6) (Figure 1A, B and C).
Lemmel syndrome, in which the primarily intrapancreatic portion of the common bile duct is compressed or obstructed by a duodenal diverticulum, was first described in 1934 (1). Several cases have been reported in the literature (7-10). Love et al. reviewed 222 articles with similar findings in a recent study, ultimately identifying 16 cases that satisfied their PubMed search criteria. Among these 16 cases, only two patients underwent transhepatic cholangiography: One due to a prior history of Billroth II gastrojejunostomy and the other due to an inability to visualize the significant duodenal papilla endoscopically (6).
In addition to obstruction, the location of the diverticulum may also lead to a malfunction of the sphincter of Oddi (7-9, 11). Most cases present with abdominal pain or symptoms of cholangitis or pancreatitis, in addition to jaundice, and may mimic a periampullary tumor. Diagnosis can be made by imaging, including CT, MRI, or ERCP, which would demonstrate the compression of the CBD and the absence of stones or tumors. Endoscopic sphincterotomy is the preferred method of treatment for biliary obstruction in these cases; however, a surgical approach with diverticulectomy might be indicated in recurrent cases. Although surgical excision is curative, elective surgery for asymptomatic cases is not recommended (11, 12).
In the study published by Alzerwi, PTC was performed after an unsuccessful ERCP, and it was shown that the patients' complaints disappeared after decompression of the biliary system (13).
In our case, the papilla was draining into the diverticulum, and the bezoar had grossly obstructed the papilla, rendering the ERCP unsuccessful. PTC drainage was performed, which also confirmed the obstruction. After bilirubin levels and liver enzymes returned to normal, as well as the white blood counts, the patient underwent diverticulectomy and was discharged uneventfully.
In conclusion, Lemmel syndrome is a rare condition that may cause acute abdomen with obstructive jaundice. Increased awareness and familiarity with imaging can aid in distinguishing the diverticulum from tumors and save the patient from unnecessary surgeries.