1. Background
Solitary fibrous tumors (SFT), previously known as hemangiopericytoma, are rare soft tissue neoplasm with an age-adjusted incidence rate of 0.98 per million individuals (1, 2). Although the clinical course of most SFTs is benign, up to 35% of cases exhibit metastasis, resulting in a poor prognosis, with a median overall survival (OS) of 8.4 months and a 5-year OS of 11% (3). Hepatic metastases account for 41% of SFT metastases and can be managed with surgery, radiation therapy, chemotherapy, and transarterial chemoembolization (TACE). However, the proper management of hepatic metastasis of SFT is yet to be established because of its rarity of cases (4).
Transarterial chemoembolization is an established as one of standard treatment for hepatocellular carcinoma (5) and has demonstrated survival benefits in patients with hepatic metastasis of colorectal cancer and neuroendocrine tumors (6). However, to date, there are a limited number of reports on the efficacy of TACE and transarterial embolization for hepatic metastasis of SFT (4, 7).
2. Objectives
Herein, we report the clinical results of 13 TACE sessions for hepatic metastasis in 5 patients with SFT.
3. Patients and Methods
3.1. Patients
This study was approved by the Institutional Review Board of Seoul National University Hospital (No. 2105-078-1218). The requirement for patient consent was waived due to the retrospective nature of the study. Patients who underwent TACE for hepatic metastasis of SFT at our medical center between May 2005 and April 2021 were included. Patients’ medical records, including primary tumor location, history of previous treatments, and clinical symptoms, were reviewed. The number, size, and imaging characteristics of hepatic metastases were analyzed using pre- and post-treatment computed tomography (CT) or magnetic resonance imaging.
3.2. Transarterial Chemoembolization
Transarterial chemoembolization was performed by five interventional radiologists with > 4 years of clinical experience. A maximum dose of 50 mg of doxorubicin was mixed with 10 cc of iodized oil (Lipiodol; Laboratoire Guerbet, Aulnay-sous-Bois, France) to form an emulsion. The oil emulsion was injected via the target hepatic artery as selectively as possible, using a 1.7-F or 2.0-F microcatheter (Progreat lambda and Progreat alpha; Terumo, Tokyo, Japan). Selective TACE was defined as catheterization of the tumor-feeding vessel or the subsegmental level of the hepatic artery. The target artery was additionally embolized with gelatin sponge particles (GSP) of 150 - 350 μm (EGgel; Engain Co., Ltd., Seongnam, Korea); nonspherical polyvinyl alcohol (PVA) of 45 - 150, 150 - 250, and 250 - 355 μm (Contour; Boston Scientific, Marlborough, MA, USA); or trisacryl gelatin microspheres of 100 - 300 μm (Embosphere; Merit Medical Systems, Inc., South Jordan, UT, USA). In cases of significant tumor burden, the procedure was performed on the left and right sides in separate sessions within a 1-month interval.
3.3. Response Analysis
Radiological assessments were performed within 3 months post-treatment to evaluate changes in the size and contrast enhancement of the metastases. The response to TACE was evaluated using the modified response evaluation criteria in solid tumors (mRECIST) (8). Subsequent follow-up images and information were collected to evaluate the progression-free survival (PFS) and OS. PFS was defined as the period from each TACE session to the identification of disease progression, such as tumor growth or the presence of new intrahepatic or extrahepatic lesions. OS was defined as the period from the first TACE to the date of death or last follow-up.
3.4. Follow-Up and Adverse Events
Computed tomography follow-up was conducted 2 - 3 months post-treatment, and patients proceeded with their next scheduled TACE. Treatment was aborted if TACE provided no survival benefit. In case of a partial response to treatment, the next TACE was performed when the tumor size regressed to its original size. Information on survival was also collected. Complications were assessed on the basis of patient symptoms and laboratory findings.
4. Results
Five patients (one male and four female, average age: 59.2 ± 8.4 years) were included (Table 1). The primary origin sites of the SFT were brain (n = 4) and peritoneum (n = 1). The hepatic metastases were identified during follow-up assessments 2 - 8 years postoperatively. There were no apparent symptoms of hepatic metastasis. Hepatic metastasis was identified as a well-defined hypervascular tumor with multinodular patterns on imaging in all patients. One of the five patients had hepatic metastasis only, while the other four had metastases to multiple organs, including the lungs, bone, pancreas, and spine. All five patients had hepatic metastases in both lobes of the liver. Average number and size of the tumors were 8.3 ± 6.3 (3 - 21) and 3.1 ± 4.2 (3.0 - 12.9) cm. TACE was performed 13 times in these five patients, of which 7 were selective TACE at the subsegmental and segmental artery levels and six non-selective TACE at the sectional and lobar artery levels. In 8 out of the 13 sessions, GSP was used as the sole embolic agent, while in 3 sessions, both GSP and PVA were employed. In one session, GSP and trisacryl gelatin microsphere were utilized together, and in the remaining session, only trisacryl gelatin microsphere was employed.
| Pt No. | Sex/age (y) | Primary location | Time to hepatic metastasis (y) | Biopsy confirm | Metastasis in other organ | Other therapy | TACE session | Hepatic metastasis | Selectivity | Response (mRECIST) | PFS (mon) | OS (mon) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Size (cm) | No. | ||||||||||||
| 1 | M/51 | Brain | 6 | No | Spine | None | 1 | 3.0 | 3 | Selective | PR | 3 | 18 |
| 2 | 3.0 | 3 | Selective | SD | 2 | ||||||||
| 3 | 3.6 | 4 | Selective | PD | 0 | ||||||||
| 2 | F/72 | Perito-neum | 7 | Yes | Lung | Radiation therapy | 1 | 11.3 | 13 | Nonselective | PD | 0 | 38 |
| 2 | 11.8 | 17 | Nonselective | PD | 0 | ||||||||
| 3 | 12.6 | 21 | Nonselective | PD | 0 | ||||||||
| 3 | F/61 | Brain | 8 | Yes | Pancreas, bone | Pazopanib | 1 | 6.4 | 3 | Selective | PR | 8 | 44 |
| 2 | 5.7 | 3 | Selective | SD | 1 | ||||||||
| 4 | F/52 | Brain | 2 | Yes | None | None | 1 | 12.5 | 5 | Nonselective | SD | 1 | 35 |
| 2 | 12.7 | 5 | Nonselective | SD | 1 | ||||||||
| 3 | 12.9 | 5 | Nonselective | PD | 0 | ||||||||
| 5 | F/60 | Brain | 5 | No | Lung, bone | None | 1 | 4.4 | 11 | Selective | PR | 8 | 28 |
| 2 | 4.8 | 15 | Selective | PR | 6 | ||||||||
Patient and Tumor Characteristics, and Results of Transarterial Chemoembolization
Of the 13 sessions, four sessions (30.8%) satisfied the definition of partial response (PR) (Figure 1), 4 sessions corresponded to stable disease (SD) (30.8%), and the remaining 5 sessions corresponded to progressive disease (PD) (38.5%). All PR sessions were performed with selective catheterization at the subsegmental level and complete tumor devascularization was achieved. When analyzed for selectivity, selective TACE was performed in seven sessions, of which 4 sessions showed PR (57.1%), one showed SD, and two showed PD.
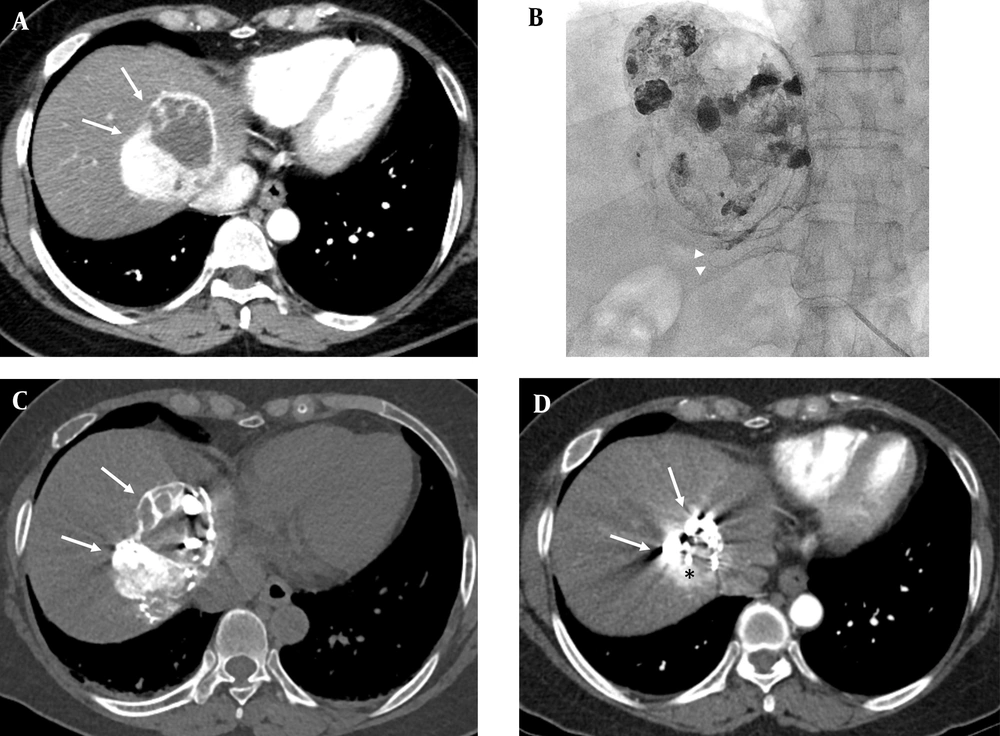
A 61-year-old female patient underwent surgery and radiation therapy for the solitary fibrous tumor in her brain 8 years ago. A, The arterial phase on computed tomography (CT) shows a 6.4 cm sized hypervascular tumor at the dome area of segment eight of the liver (arrows), which was biopsy proven as a hepatic metastasis from solitary fibrous tumor (arrows); B, Superselective catheterization of the tumor feeder was performed using a microcatheter (arrow heads). Transarterial chemoembolization was performed using adriamycin 30 mg, Lipiodol 8 cc, and gelatin sponge particles; heterogenous lipiodol uptake was observed in the post fluoroscopy image; C, Postprocedural lipiodol CT showed compact lipiodol uptake in the enhancing portion of the tumor (arrows); D, In the arterial phase of the follow-up CT 4 months after the procedure, the size of tumor decreased by 23.4% from 6.4 cm to 4.9 cm; however, arterial enhancement remained (arrows), which suggests that the lesion is a viable tumor (black asterisk).
The mean PFS was 2.3 (0 - 8) month, and the mean PFS for PR sessions was 6.3 (3 - 8) months. The mean OS was 32.7 (18 - 44) months. No severe complications were observed after the treatment. Of the 13 sessions of TACE, abdominal pain occurred after three procedures, nausea and fever occurred once, and all symptoms improved with symptomatic treatment.
5. Discussion
Transarterial chemoembolization was safely administered to all patients. The outcome of this study fell short of expectations, with PR in 30.8%. Selective TACE with complete devascularization resulted in improved PR rates (57.1%), albeit in a few patients. All patients had multiple hepatic metastases, and most of the patients had metastases in multiple organs. This advanced disease state may limit the therapeutic effect of TACE. However, TACE would be helpful for local tumor control in cases with a small number of tumors, where selective TACE can be implemented.
So far, only one study, by Velayati et al., has reported the outcomes of hepatic artery embolization for liver metastasis of SFT in 12 patients with 33 sessions (7). In this study, bland embolization of the hepatic artery was performed using a permanent embolic material. The results revealed an overall response rate of 84% (42% CR and 42% PR). The median OS after embolization was 4 years (95% confidence interval [CI], 2.3 - 5.2), and the mean PFS was 6 months (95% CI, 3.2 - 7.1). In this study, 41.7% of patients had a single lesion, and the endpoint of embolization was complete stasis of the treated vessel. Although the number of patients in this study was small, the results showed that selective embolization with complete devascularization could help control the hepatic metastases of SFT.
In this study, doxorubicin was used as a chemotherapeutic agent for metastatic SFT. However, there is currently no standard chemotherapy regimen for hepatic metastasis of SFT (9). In a previous study, doxorubicin- and dacarbazine-based treatments were tested in 13 patients; 46% had PR and 15% had SD according to the RECIST criteria (10). In another study with 13 patients, pazopanib, which is a tyrosine kinase inhibitor targeting the vascular endothelial growth factor receptor (VEGFR) and platelet-derived growth factor receptor (PDGFR), achieved a PR and SD of 9% and 73%, respectively, using the RECIST criteria, and 46% and 36%, respectively, using the Choi criteria (11). Sunitinib, another tyrosine kinase inhibitor was used to treat 31 patients and reportedly achieved 6% RECIST PR and 52% SD (12). Recently, a case report described the benefit of targeting intratumoral lactic acidosis TACE for non-islet cell tumor hypoglycemia secondary to hepatic metastasis of SFT (13). Therefore, improved chemotherapeutic agents and endovascular therapies, such as TACE, may have a synergistic effect, and further studies are needed to elucidate their precise role.
This study has several limitations. There are small sample size of 5 patients, retrospective design, and the absence of statistical analysis and a control group, which weaken the level of medical evidence. Further study with a relatively large number of patients would be helpful to verify the efficacy and survival benefit of TACE for hepatic metastasis of SFT.
In conclusion, this report suggests that TACE might be a potentially safe treatment option for hepatic metastasis of SFT, and it could offer a benefit in controlling local tumor growth in cases where selective TACE is applicable.