1. Background
Over the past few decades, there have been substantial advancements in the screening, diagnosis, and treatment of breast cancer. Despite these improvements, approximately 12% of women diagnosed with this disease still develop metastases. This development can significantly impact breast cancer mortality rates, as the axillary lymph nodes are the primary pathway for the systemic spread of cancer cells. In recent years, axillary staging via sentinel lymph node biopsy (SLNB) has emerged as the preferred method for early nodal staging of breast cancer (1). However, the findings from extensive randomized trials have shown that in addition to its high cost and invasive nature which can lead to several complications, SLNB has a false negative rate of approximately 12%, particularly in nodes that are completely infiltrated (2). While numerous studies have highlighted the potential of ultrasound in reducing the high rate of false negative results (3), there are currently no established ultrasound criteria for this purpose (3).
Moreover, the recently conducted sentinel node versus observation after axillary ultrasound (SOUND) trial, along with the earlier Z0011 clinical trial, showed no improvement in survival rates or long-term outcomes for certain patients with early-stage breast cancer who underwent axillary lymph node dissection (ALND). Some research even proposed the idea of marking metastatic nodes with clips and reassessing the axilla by excising these nodes following neoadjuvant treatment (4). As the findings from these trials are incorporated into clinical practice, the significance of residual axillary disease and the role of imaging in the preoperative staging of the axilla continue to evolve.
Ultrasound has become the most prevalent method for evaluating the axilla in patients with breast cancer due to its cost-effectiveness and non-invasive nature. It identifies lymph node involvement by assessing specific parameters, such as the absence of hilum or the presence of focal or diffuse cortical thickening. Other proposed ultrasound criteria include the long-axis and short-axis diameters of the lymph node, the size of the primary tumor, the number of visible lymph nodes, and the presence of focal or diffuse cortical changes (2, 5-7), (8-11).
The majority of research to date has primarily concentrated on the sensitivity of ultrasound findings, establishing criteria to rule out clinically significant axillary lymph node involvement. However, the use of ultrasound parameters to pinpoint patients who might not need ALND has proven to be challenging. Despite their sensitivity, these ultrasound parameters have not supplanted SLNB or fine needle biopsy (FNB). As of now, there are still no standard ultrasound criteria in place for this purpose (3). In this study, we sought to approach the issue from a different perspective. Our goal was to confirm the presence of metastatic adenopathy using sonographic parameters. As such, we opted for cut-off values that exhibit high specificity and focused solely on the lymph nodes that appeared most abnormal. Our hypothesis was that the application of ultrasound criteria with high specificity could provide clinicians with assurance of lymph node metastasis. This could be particularly useful in special cases where SLNB results are falsely negative. Furthermore, these criteria could help identify patients who could be potentially excluded from a treatment course directed by the Z0011 trial. In certain patients, this could even eliminate the need for further investigations, including SLNB. The use of highly specific cutoff values could indeed prove beneficial for future research, particularly when devising targeted treatment strategies for axillary metastasis.
2. Objectives
This study aimed to define highly specific ultrasound parameters to ascertain axillary lymph node involvement in selected patients who need ALND.
3. Patients and Methods
3.1. Study Setting and Population
In this prospective cross-sectional study, patients admitted to Omid Hospital in Mashhad, Iran from 2018 to 2022 were included through non-probability purposive sampling. These patients had a confirmed diagnosis of breast cancer, as verified by an oncologist, and a normal clinical examination of the axilla. Generally, Omid Hospital is a tertiary educational oncology center in the northeast of Iran and a referral center with various patients from nearby cities and provinces. Meanwhile, patients undergoing neo-adjuvant treatments and those with no visible lymph nodes in axillary ultrasound examinations were excluded.
3.2. Sample Size
The sample size (256 patients) was determined based on the area under the curve (AUC) for cortical thickness in patients with metastatic breast cancer, according to a study by Farrokh et al. (12). The study was designed with an alpha level of 0.05 and a power of 90%, taking into account a potential dropout rate of 10%.
3.3. Data Collection
Data were gathered using a checklist including demographic characteristics and ultrasound findings. All participants underwent B-mode ultrasound examination of the breast and axilla by a single experienced radiologist with 15 years of experience in the field of breast imaging after collection of demographic data. Both breasts and axilla were scanned bilaterally using a high-resolution ultrasound scanner (12-MHz linear-array transducer, Samsung WS80, Korea, class C Esaote, Italy) under the same standard setting. The radiologist performing the ultrasound examinations was blinded to the patients' demographic, clinical, and histological information. The intra-rater agreement for ultrasound examinations was calculated in 10 patients. The interclass correlation coefficient (ICC) was measured to be 0.9.
For the axillary evaluation, patients were placed in a supine oblique position, with their arms abducted and externally rotated above their heads. Both axillary regions were examined in longitudinal and transverse planes. The lymph node with the most atypical cortex, appearing most suspicious, was selected. If all lymph nodes appeared normal, the most prominent ipsilateral lymph node in the lower axilla was chosen for further evaluation. Additionally, the most prominent normal-appearing lymph node on the contralateral side was assessed.
In order to identify the optimal cut-off points for ultrasound parameters in detecting lymph node involvement, several measurements were taken, including the tumor size (greatest diameter), the cortical thickness of the axillary lymph node, the short axis diameter of the node, and the number of lymph nodes with a cortical thickness of ≥ 3 mm. These measurements were then compared with the histological diagnosis following SLNB or ALND. After SLNB and subsequent ALND if necessary, the patients’ primary tumor histology, lymphovascular invasion, number of dissected lymph nodes, and number of involved lymph nodes were recorded. Lymph node involvement was defined by the presence of micro (< 2 mm) or macro (> 2 mm) metastasis in histology.
3.4. Ethical Considerations
Informed consent was obtained from all patients before participating in this study. The Research Ethics Committee of Mashhad University of Medical Sciences approved the present study under the code of IR.MUMS.MEDICAL.REC.1399.532.
3.5. Statistical Analysis
Data were analyzed using IBM SPSS version 25 (IBM SPSS statistics for Windows, version 25.0. Armonk, NY: IBM Corp). All characteristics of the patients were described as mean ± standard deviation (SD), median, and interquartile range (IQR) or frequency. Normal distribution of data was verified using Kolmogorov-Smirnov test. The relationship between qualitative variables was evaluated using chi-square test or Fisher’s exact test. The optimal cut-off values for ultrasound criteria were determined using a receiver operating characteristic (ROC) curve analysis, AUC, and the Youden Index. For all statistical analyses, a P-value of less than 0.05 was considered statistically significant. The AUC values of 0.9 to 1 indicated a high diagnostic accuracy, values of 0.8 to 0.9 represented a very good diagnostic power, values of 0.7 to 0.8 indicated a good diagnostic power, and values of 0.7 to 0.6 represented an adequate diagnostic power. A P-value < 0.05 was considered statistically significant.
4. Results
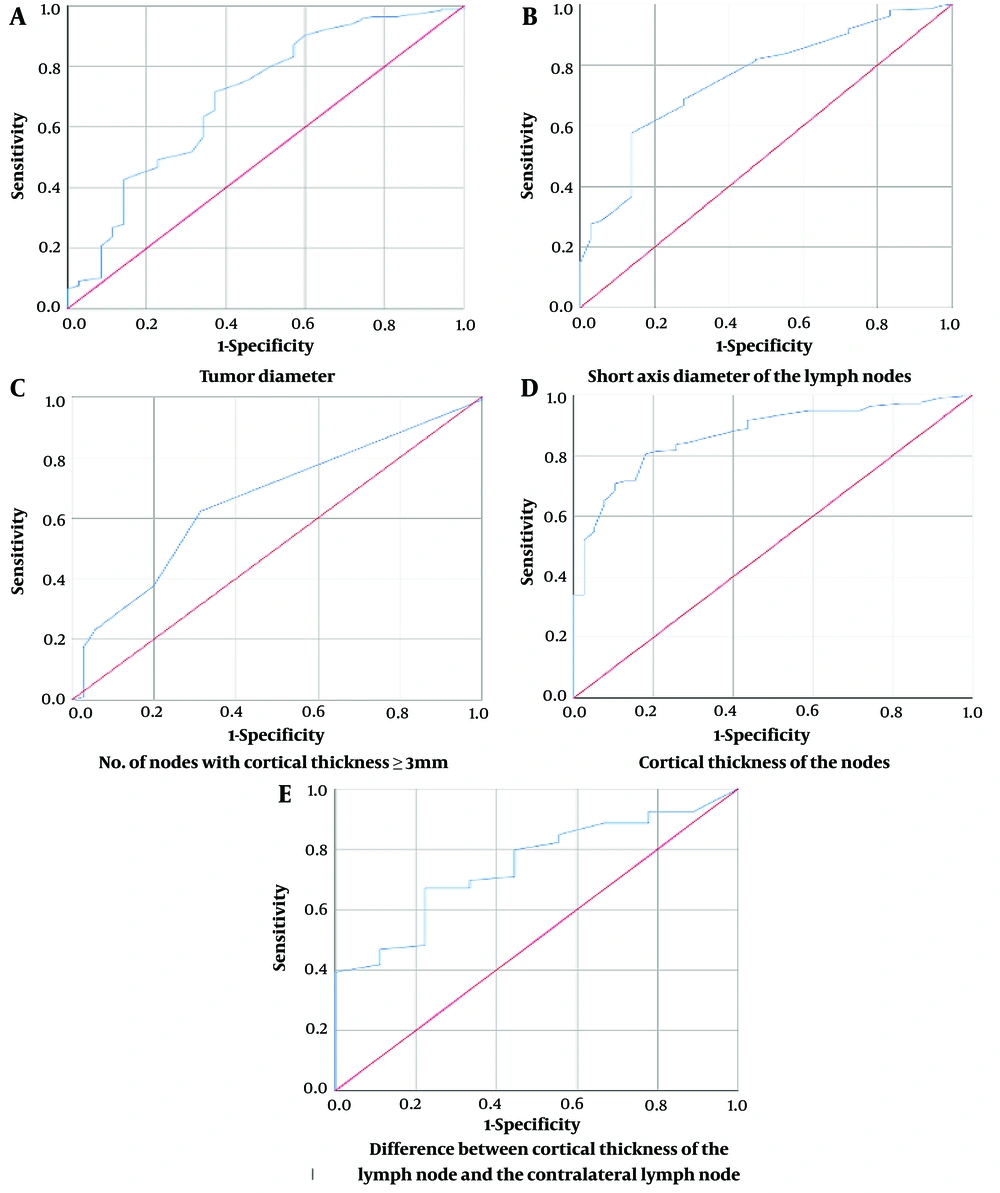
A total of 256 women, with an average age of 46.41 ± 10.77 years, participated in this study. The AUC and optimal cut-off values for each of the ultrasound parameters measured are presented in Figure 1 and Table 1. The average of the maximum tumor diameters among the patients was found to be 29.1 mm. Table 2 represents the T staging, number of lymph nodes, and age range of the participants.
| Parameter | AUC (95% CI) | P-Value | Cut-off Point | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy (%) |
|---|---|---|---|---|---|---|---|---|
| Tumor diameter (mm) | 0.700 (0.599 - 0.801) | < 0.01 | 50.5 | 91 | 97 | 90 | 97.38 | 95.62 |
| Number of nodes with cortical thickness ≥ 3 mm | 0.660 (0.568 - 0.752) | < 0.01 | 3.5 | 23.2 | 94.3 | 53.06 | 80.33 | 77.58 |
| Short axis diameter of lymph node (mm) | 0.756 (0.675 - 0.836) | < 0.01 | 12.2 | 28.6 | 94.4 | 58.7 | 81.38 | 78.78 |
| Cortical thickness of lymph node (mm) | 0.869 (0.817 - 0.922) | < 0.01 | 6.05 | 56 | 95 | 77.10 | 87.92 | 85.95 |
| Difference in cortical thickness with contralateral node (mm) | 0.745 (0.605 - 0.884) | < 0.01 | 4.6 | 40 | 100 | 100 | 84.80 | 86.2 |
Abbreviations: ROC, receiver operating characteristic; AUC, area under the curve; CI, confidence interval; PPV, positive predictive value; NPV, negative predictive value; mm, millimeters.
| Age Range (y) | No. (%) |
|---|---|
| ≤ 40 | 76 (29.6 ) |
| 40 - 60 | 147 (57.4) |
| 60 < | 33(12.8) |
| Tumor Size | |
| T1 (0 - 2 centimeters) | 75 (29.2) |
| T2 (2 - 5 centimeters) | 154 (60.1) |
| T3 (> 5 centimeters) | 27 (10.5) |
| Number of lymph nodes | |
| < 3 | 164 (64) |
| ≥ 3 | 92 (35.9) |
According to the ROC curves, tumor diameters above 50.5 mm could detect lymph node involvement with 97% specificity and 91% sensitivity. A cut-off point of 12 mm for the short axis diameter (SAD) of the lymph node on ultrasound was associated with 95% specificity. A cortical thickness above 6 mm could detect lymph node involvement with 95% specificity and 56% sensitivity. Likewise, a difference of > 4.5 mm between the cortical thickness of the suspicious lymph node and that of the contralateral normal lymph node had 100% specificity for diagnosing lymph node involvement.
Based on the findings, having more than three ipsilateral lymph nodes with a cortical thickness of ≥ 3 mm showed 94.3% specificity for diagnosing axillary lymph node involvement. Among our patients, 49 (20.5%) exhibited lymphadenopathy with a cortical thickness of ≥ 3 mm in ≥ 3 lymph nodes. According to these criteria, 129 (56%) patients had metastatic lymphadenopathy with a sensitivity of 64.6% and a specificity of 91.4% and did not benefit from SLNB. Figures 2 - 4 demonstrate several ultrasound features of metastatic axillary lymph nodes and primary tumors.
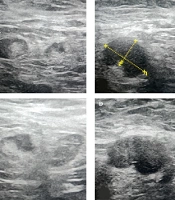
A, ultrasound image of the upper outer left breast in a 44-year-old female shows a 5.2 cm irregular hypoechoic mass (calipers) which was an invasive ductal carcinoma at biopsy; B, US image of the left axilla shows a suspicious lymph node with complete loss of the fatty hilum. Sentinel lymph node biopsy (SLNB) confirmed the presence of metastatic disease and axillary lymph node dissection (ALND) was performed at the time of mastectomy.
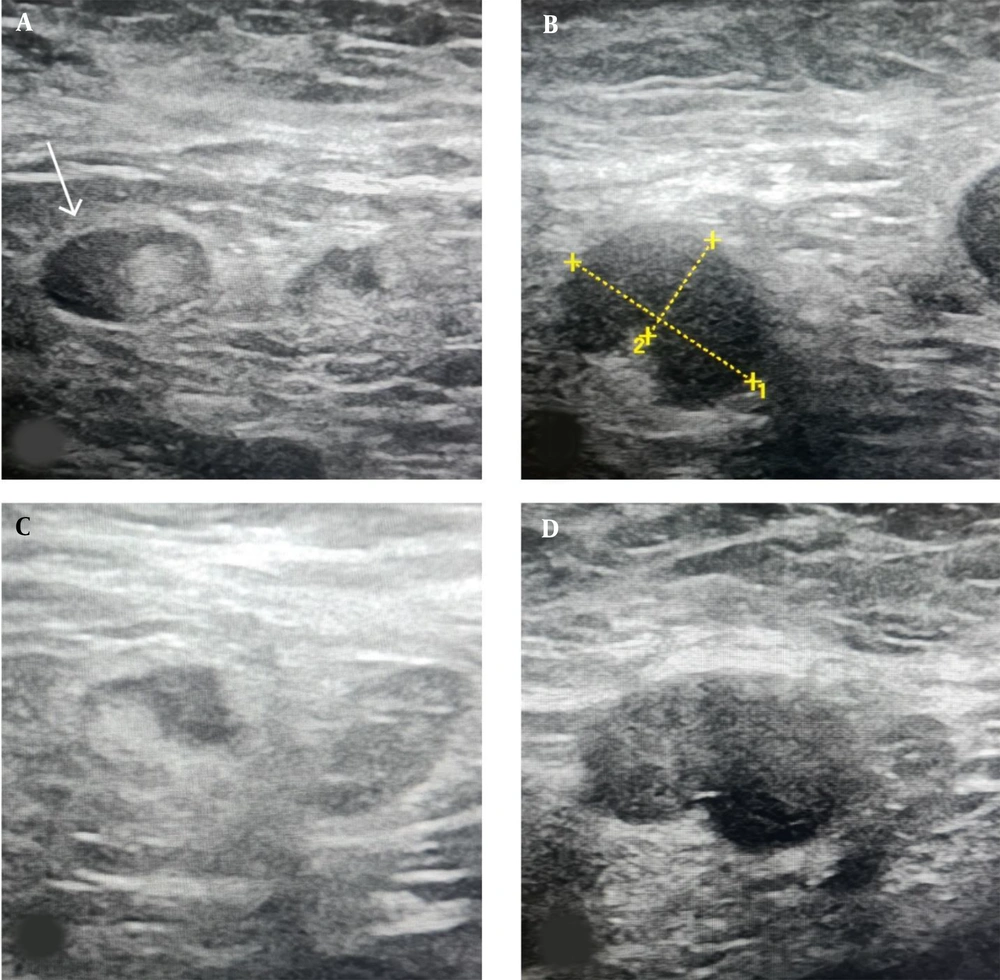
Ultrasound Features of metastatic axillary lymph nodes: A, US image shows a metastatic axillary node with a focally eccentric thickened cortex (arrow); B, US image shows a 16 mm metastatic axillary node with an abnormal round shape, partial loss of the fatty hilum, and a 9 mm thickened cortex (calipers2); C, US image shows another infiltrated lymph node with an abnormal focal bulge in the cortex; D, Another biopsy-proven metastatic lymph node in a 63-year-old female patient with invasive ductal carcinoma (IDC). Ultrasound image shows an oval-shaped, marked hypoechoic and enlarged lymph node with absent fatty hilum.
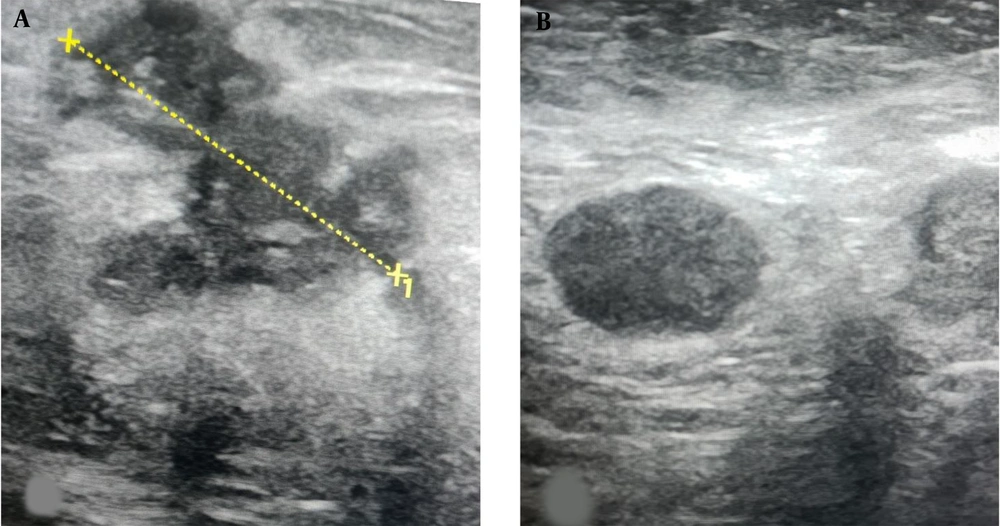
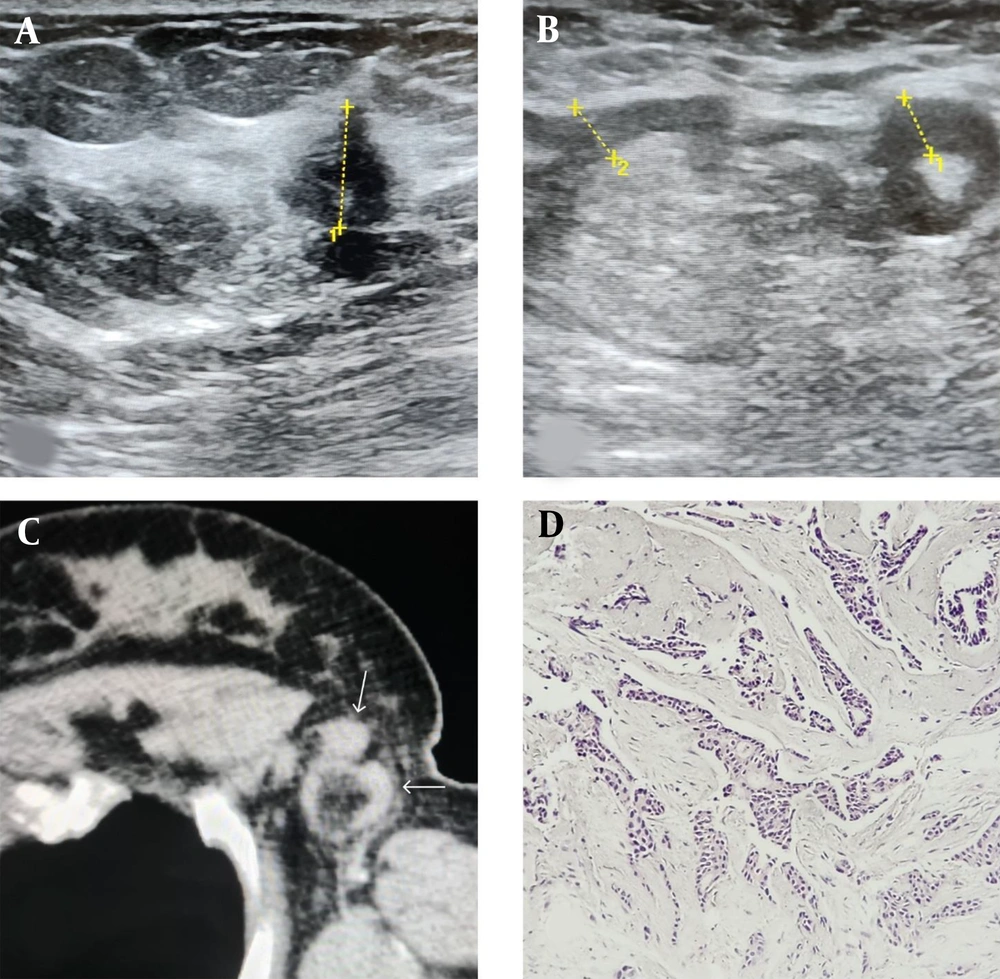
A, ultrasound image of the upper outer left breast in a 42-year-old female shows a 13 mm hypoechoic irregular mass with an angular margin (calipers 1); B, ultrasound image of the axilla in the same patients shows two suspicious lymphadenopathies with uneven thick cortexes measuring 6 mm (calipers 1) and 5.7 mm (calipers 2); C, CT scan image of the axilla in the same patient shows the two suspicious nodes with fatty hilum (white arrows); D, the pathologic specimen demonstrates malignant carcinomatous proliferation evident as small irregular cell clusters and atypical ductal cells compatible with low grade invasive ductal carcinoma
5. Discussion
Traditionally, ALND has been utilized as a method to evaluate axillary lymph nodes. While the primary aim of this procedure is to decrease the risk of axillary recurrence (12, 13), it is associated with significant morbidity and does not provide therapeutic benefits for all patients. According to recent studies, following the publication of clinical trials, including Z0011, many surgeons have begun to reconsider the use of ALND in certain patients with metastatic axillary adenopathy (14). Although intraoperative SLNB has widely replaced ALND as a primary diagnostic modality in many centers (5, 15), it has certain drawbacks, including a high cost, invasiveness, limited accessibility to radio-labelled colloids in some countries, and a 12% false negative rate in completely infiltrated nodes that do not take up the sulfur colloid (16, 17). While procedures, such as core needle biopsy and fine needle aspiration (FNA) are effective methods to decrease the number of SLNBs, they are still invasive in nature. There is also a risk that these procedures could potentially damage the afferent lymphatic vessels, which could subsequently result in a decreased detection rate by SLNB (2).
Ultrasound examination is a prevalent initial assessment method for patients with breast cancer. It is a cost-effective and reliable technique for determining the disease stage. As suggested by Alvarez et al., using morphological criteria, ultrasound can accurately predict lymph node involvement in breast cancer. This allows patients with positive ultrasound results to be directly referred for ALND (5). While some studies have highlighted the significance of false negative results in preoperative ultrasound, there is evidence suggesting that an advanced nodal stage is linked with lower false negative rates (18).
While axillary dissection remains the standard care and is unavoidable for many patients (19), it is important to consider the clinical implications of emerging evidence. This includes findings from the SOUND clinical trial and the Z0011 trial, which suggest that ALND may not be routinely necessary for all patients with positive sentinel lymph nodes. In other words, the presence of any small cancerous deposit in the axilla does not automatically necessitate ALND in all patients. In the present study, we examined different ultrasound parameters to determine the cut-off values with high specificity by which we can diagnose severe lymph node involvement with high certainty. These parameters could potentially eliminate the need for SLNB, aiding clinicians in treating these patients with caution in their future management, even in cases of negative SLNB results. Furthermore, these parameters could assist surgeons in identifying patients who may not benefit from a treatment course directed by the Z0011 trial.
The first important feature evaluated in our study for predicting axillary lymph node involvement in breast cancer was the size of the primary tumor (20). Previously, Mainiero et al. evaluated lymph node appearance and FNA results in 224 patients with breast cancer and revealed that ultrasound-guided FNA of lymph nodes was most useful when the tumor size was > 2 cm (21). Similarly, in a study on 3,115 patients with breast cancer, those with a tumor size > 2 cm were more likely to have sentinel lymph node involvement (22). We found that a tumor size > 50.5 mm had a 97% specificity for predicting lymph node metastasis, with an accuracy of 95.62% and a negative predictive value (NPV) of 97.38%. These results align closely with the tumor size used in the staging of breast cancer, where a tumor size > 5 cm is considered stage T3 (23).
While the literature indicates that a lymph node with a short axis diameter (SAD) of ≥ 10 mm is considered abnormal, numerous studies have demonstrated that benign and malignant nodes can have similar mean diameters. Therefore, size alone may not be a reliable indicator of metastasis (24, 25). One systematic review reported a sensitivity of 49 - 87% and a specificity of 55 - 97% for ultrasound examination in detecting lymph node metastasis based solely on size (5). Meanwhile, by using morphological criteria, the sensitivity was measured to be 26 - 76%, and the specificity was 88 - 98% (26). Using a short axis diameter of 12.7 mm, we could predict metastatic involvement with 97.2% specificity, 80.81% accuracy, and 81.70% NPV. However, some studies suggest that size cannot be a reliable parameter, as reactive lymph nodes may be larger than metastatic ones. Therefore, measuring the size alone is not recommended for diagnosing a metastatic disease (27).
As metastatic cells represent a centrifugal pattern of implantation in lymph nodes, cortical changes may be more important than other ultrasound indices (6, 9). It has been shown that a cortical thickness of > 3 mm is the most useful indicator of malignancy in clinical practice (21). However, defining a cut-off point for cortical thickness mainly depends on the purpose of the ultrasound examination (11). Our study identified a cortical thickness of 6 mm as the optimal cut-off point, with a sensitivity of 56% and a specificity of 95%. This suggests that setting a 6 mm threshold for lymph node cortical thickness significantly reduces the false positive results. Furthermore, patients exhibiting this abnormal ultrasound finding may not derive substantial benefit from SLNB or FNA. Our findings align closely with those of a systematic review by Alvarez et al., which reported that ultrasonography alone could detect approximately half of the axillary metastases with a specificity of 96.5%. While this study also applied morphological criteria, it was evident that lymph nodes with thicker cortexes represented more significant morphological changes. Consequently, the authors suggested that patients with these characteristics could be directed towards axillary dissection (5).
Similarly, Farrokh et al. showed that a cortical thickness of > 5 mm was the best cut-off point, with 80% sensitivity and 94 - 100% specificity (12). A retrospective study conducted in 2022 assessed 336 breast cancer patients and showed that cortical parameters, including a cortical thickness of > 3 mm on ultrasound, yielded sensitivity, specificity, positive predictive value (PPV), NPV, and accuracy of 83%, 62%, 59.2%, 54.8%, and 79.1%, respectively, for detecting lymph node metastases. The authors used a smaller cut-off value compared to our study, which accounts for the higher sensitivity. Interestingly, their findings suggested that the performance of magnetic resonance imaging (MRI) was only marginally superior to axillary ultrasound (28).
Our study determined that the optimal cut-off point for cortical thickness difference, when compared with contralateral normal nodes, was 4.6 mm. This value, which demonstrated 100% specificity, suggests that a cortical thickness difference of > 4.5 mm can definitively indicate the involvement of ipsilateral axillary nodes. Additionally, the detection of ≥ 3 axillary lymph nodes with a cortical thickness ≥ 3 mm could diagnose involvement with a specificity of 94.3% and an NPV of 85.6%. These findings are consistent with the pathological nodal (pN) staging of breast cancer according to the National Comprehensive Cancer Network (NCCN) guidelines, where the presence of metastasis in 1 - 3 axillary lymph nodes is considered pN1, and metastasis in 4 - 9 axillary lymph nodes is considered pN2 (23). In line with these results, a decrease in the rate of false negative results has been observed in breast cancer patients with ≥ 3 sentinel nodes excised (17). In addition, Imai et al., in a retrospective study of 470 patients with breast cancer, observed that those with three lymph nodes with SAD >10 mm had metastatic involvement (8).
In summary, three ultrasound findings can detect metastasis to axillary lymph nodes with a specificity of > 95%. These include axillary lymphadenopathy with a cortical thickness exceeding 6 mm, a difference > 4.5 mm between the cortical thickness of the suspected lymph node and the contralateral lymph node, and/or the presence of ≥ 3 lymph nodes with a cortical thickness of ≥ 3 mm. As such, ultrasound, being a readily available alternative method, can detect lymph node metastasis in over 50% of patients. Therefore, it appears that patients exhibiting these abnormal ultrasound findings may not derive significant benefit from SLNB, FNA, and/or biopsy.
Our study had a few limitations. First, it was carried out in a referral oncology hospital and utilized non-probability purposive sampling. A significant number of patients who visited our clinic were already in the advanced stages of breast cancer. As a result, the study population exhibited a high prevalence of lymph node involvement. Therefore, further research with a multicenter design and using a random sampling method is required to confirm our results. Second, our study did not incorporate certain parameters derived from cortical thickness (such as eccentricity, irregularity, and focal cortical thickness), flow parameters, or the BIRADS classification in the lymph node analysis. Including these parameters might have enhanced the specificity of the ultrasound criteria, but it would have also added a layer of complexity to the analysis. Indeed, incorporating these parameters in future studies and including patients without visible lymph nodes in axillary ultrasound examinations could indeed enhance the generalizability of the findings.
While large clinical trials, including the SOUND Trial (29), the Intergroup-Sentinel-Mamma (INSEMA) Trial (30), the Dutch BOOG 2013-08 Trial in Europe, and the NAUTILUS study for the Asian population, have evaluated the outcomes of omitting SLNB (31), undoubtedly, the use of ultrasound criteria with high specificity can significantly aid clinicians in accurately predicting lymph node metastasis prior to surgery. The implications of this finding are substantial and should not be overlooked.
In conclusion ultrasound, being a cost-effective, accessible, and non-invasive diagnostic tool, can serve as a supplementary method to SLNB or even an alternative to it in detecting lymph node involvement in over 50% of patients. The ipsilateral presence of > 3 lymph nodes with a cortical thickness of ≥ 3 mm, a difference of ≥ 4.5 mm between the cortical thickness of the suspected lymph node and the contralateral lymph node, a cortical thickness of ≥ 6 mm are ultrasound findings that can detect metastasis to axillary lymph nodes, with a specificity ranging from 95% to 100%.