1. Background
Despite significant advances in the early diagnosis and treatment of non-small cell lung cancer (NSCLC), it remains one of the leading causes of cancer-related death worldwide (1). While disease stage is the most crucial determinant of survival in NSCLC patients (2-4), survival duration can vary significantly among patients with tumors at the same stage. Therefore, more accurate predictors of survival are needed for NSCLC patients.
Computed tomography (CT) integrated with positron emission tomography (PET/CT) using 18F-fluorodeoxyglucose (18F-FDG) is a molecular imaging method that provides information based on changes in tissue metabolism. In addition to its role in diagnosis and staging, PET/CT may also aid in predicting the prognosis and survival of NSCLC patients (3-5).
Several semi-quantitative parameters calculated from a numerical analysis of PET/CT images can be used for tumor quantification. These parameters indicate the metabolic activity, and thus the biological behavior, of the primary tumor. The most commonly used parameters are the maximum standardized uptake value (SUVmax) and the mean standardized uptake value (SUVmean). However, neither of these parameters represents the activity of each part of the tumor or provides information about tumor burden (6, 7). This raises questions about the efficacy of SUVmax and SUVmean for survival prediction. Although some studies have reported that tumor SUVmax may have prognostic significance in NSCLC patients (6, 8-12), other studies have not found this association (13-15).
Consequently, additional PET/CT parameters have been used to predict tumor behavior. The metabolic tumor volume (MTV) is a volumetric-metabolic indicator of metabolically active tumor volume that provides information on tumor burden. Total lesion glycolysis (TLG) is defined as the total activity in tumor tissue, representing the activity of the tumor as a whole. The TLG is calculated by multiplying the MTV by the SUVmean. Since MTV and TLG values provide both metabolic and volumetric information, they may be more useful in predicting prognosis than SUV-based values. Indeed, some studies have reported that high MTV and TLG values in all stages of NSCLC are associated with a high risk of adverse events and worse survival (6, 15-19).
2. Objectives
This study evaluated the associations of tumor SUVmax, SUVmean, MTV, and TLG, as determined from pre-treatment PET/CT images, with survival in NSCLC patients.
3. Patients and Methods
This retrospective cohort study was approved by our hospital’s Scientific Board and Ethics Committee (approval number: 05.11.2020/2020-42).
3.1. Patients
Patients diagnosed with NSCLC at our clinic between January 2019 and October 2020 were identified in the electronic database. Patients with NSCLC of any stage, who had a PET/CT scan before receiving any treatment, who were over 18 years of age, and whose images and treatment information (surgery, chemotherapy, radiotherapy, etc.) could be accessed from the electronic database were eligible for the study. To avoid errors in data analysis caused by different devices, only the 132 patients imaged using the same device in our hospital were finally included in the study.
3.2. Definitions
Tumor size was measured using CT, and lymph node involvement was determined based on the PET/CT findings. These data, along with brain magnetic resonance imaging data, were used to determine the tumor stage according to the 8th edition of the tumor, node, metastasis (TNM) staging system (20).
Computed tomography attenuation-corrected FDG-PET images were reconstructed by applying a Bayesian penalized likelihood reconstruction algorithm using Beta 400. Images were displayed in a 192 × 192 CT matrix (slice thickness, 3.27 mm), and FDG-PET scan data were accurately co-registered on a workstation using ADW software (GE Healthcare, Chicago, IL, USA). 18F-FDG-PET/CT images were reviewed by nuclear medicine physicians and a radiologist who were blinded to the clinical information. Target lesions were identified as primary tumors based on 18F-FDG uptake and anatomical location. The SUVmax, SUVmean, MTV, and TLG values of the primary tumor were calculated using the fixed threshold-based tumor segmentation method.
metabolic tumor volume represents the three-dimensional total volume measured by the region of interest (ROI) drawn around the lesion. The ROI of the primary tumor was drawn manually, and the parameters were automatically calculated by the software program of the device. A cut-off value of 42% was used in MTV calculation.
Data on variables that may affect survival (age, sex, histopathological type, disease stage) were also recorded. Patients were followed up after the date of diagnosis. Survival was defined as being alive, and survival time as the time between diagnosis and death or, for surviving patients, between diagnosis and the date of the last contact with the patient.
3.3. Statistical Analysis
The data were analyzed using IBM SPSS Statistics (version 22.0; IBM Corp., Armonk, NY, USA) statistical software. Continuous variables are presented as the mean and standard deviation (SD), and categorical variables as the number (n) and percentage (%). Spearman's correlation test was used when at least one of the variables was non-parametric or the data distribution was not normal. The survival rate was calculated using the Kaplan-Meier method.
The ability of SUVmax, SUVmean, MTV, and TLG to predict survival was determined. The Cox proportional hazards regression model was used in the univariate and multivariate analyses of survival, as it is suitable for both continuous and binary variables. Metabolic tumor volume and TLG were included in the survival analysis as continuous variables. Confounding variables, such as patient age, primary tumor diameter, and primary tumor SUVmax and SUVmean, were also analyzed as continuous variables. For categorical confounding variables {sex, tumor histologic type [squamous cell carcinoma (SSC) vs. adenocarcinoma (ACA)], disease stage} the category with the lowest risk served as the reference group.
Survival was also examined in a multivariate analysis. Confounding variables with a P-value < 0.1 in the univariate analysis were included in the multivariate analysis. In all analyses, P < 0.05 was considered statistically significant.
4. Results
4.1. General Variables
The mean age of the 132 study patients was 62.24 years (SD: 11.19); 18 (13.64%) were female, and 114 (86.36%) were male. Tumor types included SCC in 59 (44.7%) patients, ACA in 44 (33.3%) patients, and NSCLC of undetermined subtype in 29 (22%) patients. The mean diameter of the primary tumor was 5.42 cm (SD: 2.64). Lymph node metastasis was detected in 28 (21.2%) patients, 102 (77.3%) patients did not have lymph node metastasis, and the exact lymph node stage was not determined in 2 (1.5%) patients. Stage 1 disease was diagnosed in 14 (10.6%) patients, stage 2 in 5 (3.8%) patients, stage 3 in 59 (44.7%) patients, and stage 4 in 54 (40.9%) patients.
4.2. Positron Emission Tomography Variables of the Primary Tumor
The mean SUVmean of the tumors was 9.14 (SD: 4.62), and the mean SUVmax was 16.03 (SD: 8.22). The mean MTV was 60.63 cm³ (SD: 84.26), and the mean TLG was 527.66 g/ml × cm³ (SD: 603.73). The relationship between these values was analyzed using a non-parametric correlation analysis due to the non-normal distribution of the data.
A near-excellent correlation was found between SUVmean and SUVmax (rho = 0.91, P < 0.0001). The correlation between SUVmean and TLG was moderate (rho = 0.60, P < 0.0001), while the correlation between SUVmean and MTV was fair (rho = 0.28, P = 0.001). A fair and significant correlation was observed between SUVmax and MTV (rho = 0.30, P = 0.001), and a moderate and significant correlation was noted between SUVmax and TLG (rho = 0.59, P < 0.0001). An excellent and significant correlation was found between MTV and TLG (r = 0.92, P < 0.0001), and a good and significant correlation was seen between the primary tumor diameter and MTV (rho = 0.68, P < 0.0001).
4.3. Relationships Among Primary Tumor Positron Emission Tomography Variables, Lymph Node Metastasis, and Distant Metastasis
SUVmean did not significantly correlate with either lymph node metastasis or distant metastasis. The correlation between SUVmax and lymph node metastasis was poor and statistically insignificant, as were the correlations between SUVmax and distant metastasis, and between MTV and lymph node metastasis. However, despite the weak correlation between MTV and distant metastasis, the relationship showed a trend towards significance (P = 0.05). TLG was not significantly associated with lymph node metastasis or distant metastasis (Table 1).
| Variables | Lymph Node Metastasis | Distant Metastasis | ||
|---|---|---|---|---|
| Correlation Coefficient (rho) | P-Value | Correlation Coefficient (rho) | P-value | |
| SUVmax value | 0.12 | 0.17 | -0.01 | 0.89 |
| SUVmean value | 0.07 | 0.46 | -0.12 | 0.18 |
| MTV value | 0.14 | 0.11 | 0.17 | 0.055 |
| TLG value | 0.12 | 0.16 | 0.11 | 0.22 |
Abbreviations: PET, positron emission tomography; SUVmax, primary tumor maximum standardized uptake value; SUVmean, primary tumor mean standardized uptake value; MTV, primary tumor metabolic tumor volume; TLG, primary tumor total lesion glycolysis.
4.4. Survival and the Factors Affecting Survival
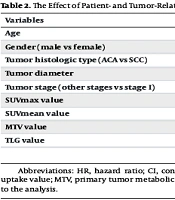
The overall 2-year survival rate of the study patients was 36.6%. The median survival time was 13 months (SD: 1.67). In the univariate analysis, age, tumor diameter, and disease stage were associated with survival. The PET parameters MTV and TLG were associated with survival, whereas SUVmax and SUVmean were not (Table 2). Therefore, both MTV and TLG were included in the multivariate analysis.
| Variables | Univariate HR (95% CI) | Univariate P-Value | Multivariate HR (95% CI) | Multivariate P-Value |
|---|---|---|---|---|
| Age | 1.03 (1.002 - 1.049) | 0.03 | 1.05 (1.02 - 1.07) | < 0.0001 |
| Gender (male vs female) | 1.29 (0.64 - 2.59) | 0.47 | NI | NI |
| Tumor histologic type (ACA vs SCC) | 0.65 (0.37 - 1.14) | 0.13 | NI | NI |
| Tumor diameter | 1.09 (1.01 - 1.18) | 0.02 | 1.06 (0.97 - 1.16) | 0.20 |
| Tumor stage (other stages vs stage I) | 2.70 (1.87 - 3.91) | < 0.0001 | 3.50 (2.28 - 5.37) | < 0.0001 |
| SUVmax value | 0.99 (0.97 - 1.02) | 0.80 | NI | NI |
| SUVmean value | 0.98 (0.94 - 1.03) | 0.51 | NI | NI |
| MTV value | 1.003(1.001 - 1.005) | 0.003 | 1.004 (1.001 - 1.008) | 0.02 |
| TLG value | 1.00(1.000 - 1.001) | 0.045 | 0.99 (0.99 - 1.0) | 0.04 |
Abbreviations: HR, hazard ratio; CI, confidence interval; SUVmax, primary tumor maximum standardized uptake value; SUVmean, primary tumor mean standardized uptake value; MTV, primary tumor metabolic tumor volume; TLG, primary tumor total lesion glycolysis; ACA, adenocarcinoma; SCC, squamous cell carcinoma; NI, not included to the analysis.
In the multivariate analysis, tumor diameter was no longer associated with survival, whereas the associations of age and stage with survival persisted. Additionally, MTV was shown to be associated with an increased risk of death. TLG tended to be associated with survival, with poorer survival observed in patients with a lower TLG value (Table 2).
The multivariate survival analysis was repeated in patient subgroups, using patient age, tumor stage, MTV, and TLG as variables. The results of these analyses are reported in the following sections.
4.5. Subgroup Analysis According to the Histopathological Type of the Tumor
In the subgroup-based multivariate analysis, MTV and TLG were not associated with the survival of SCC patients. However, both variables had a significant effect on the survival of patients with ACA. In this latter group, higher MTV levels and lower TLG levels were associated with a shorter survival time (Table 3).
| Variables | SCC | ACA | ||
|---|---|---|---|---|
| HR (95% CI) | P-Value | HR (95% CI) | P-Value | |
| Age | 1.03 (0.99 - 1.06) | 0.09 | 1.12 (1.06 - 1.19) | < 0.0001 |
| Tumor stage | 2.09 (1.23 - 3.53) | 0.006 | 8.38 (2.85 - 24.60) | < 0.0001 |
| MTV value | 0.996 (0.985 - 1.008) | 0.51 | 1.05 (1.02 - 1.09) | 0.002 |
| TLG value | 1.001 (0.999 - 1.002) | 0.40 | 0.995(0.991 - 0.999) | 0.006 |
Abbreviations: SCC, squamous cell carcinoma; ACA, adenocarcinoma; HR, hazard ratio; CI, confidence interval; MTV, primary tumor metabolic tumor volume; TLG, primary tumor total lesion glycolysis.
4.6. Subgroup Analysis According to the Presence of Lymph Node Metastasis
Among patients without lymph node metastases, neither MTV nor TLG was associated with survival. However, in patients with lymph node metastases, higher MTV and lower TLG values were associated with shorter survival (Table 4).
| Variables | Lymph Node-negative Patients | Lymph Node-positive Patients | ||
|---|---|---|---|---|
| HR (95% CI) | P-Value | HR (95% CI) | P-Value | |
| Age | 1.13 (1.04 - 1.23) | 0.003 | 1.04 (1.01 - 1.06) | 0.004 |
| Tumor stage | 5.92 (1.63 - 21.48) | 0.007 | 3.24 (1.98 - 5.30) | < 0.0001 |
| MTV value | 1.002 (0.96 - 1.04) | 0.92 | 1.005 (1.001 - 1.008) | 0.02 |
| TLG value | 0.999 (0.996 - 1.002) | 0.61 | 0.999 (0.999 - 1.000) | 0.08 |
Abbreviations: HR, hazard ratio; CI, confidence interval; MTV, primary tumor metabolic tumor volume; TLG, primary tumor total lesion glycolysis.
4.7. Subgroup Analysis According to the Presence of Distant Metastasis
Tumor stage was not included in the analysis of distant metastasis status and survival, as distant metastasis itself determines the stage. In the multivariate analysis, neither MTV nor TLG was associated with survival in patients without distant metastasis. However, in patients with distant metastasis, higher MTV and lower TLG values were associated with worse survival (Table 5).
| Variables | Distant Metastasis-negative Patients | Distant Metastasis-positive Patients | ||
|---|---|---|---|---|
| HR (95% CI) | P-Value | HR (95% CI) | P-Value | |
| Age | 1.06 (1.001 - 1.11) | 0.01 | 1.04 (1.02 - 1.07) | 0.001 |
| MTV value | 1.008 (0.99 - 1.03) | 0.39 | 1.005 (1.001 - 1.009) | 0.01 |
| TLG value | 1.000 (0.998 - 1.001) | 0.79 | 0.999 (0.999 - 1.000) | 0.047 |
Abbreviations: HR, hazard ratio; CI, confidence interval; MTV, primary tumor metabolic tumor volume; TLG, primary tumor total lesion glycolysis.
4.8. Subgroup Analysis by Disease Stage
As noted above, among patients with stage 4 disease, higher MTV and lower TLG values were associated with worse survival (Table 6). There was no association between TLG or MTV values and survival in patients with other stages.
| Variables | Stage I | Stage II | Stage III | Stage IV | |
|---|---|---|---|---|---|
| P-Value | P-Value | P-Value | P-Value | HR (95% CI) | |
| Age | 0.32 | 0.94 | 0.09 | 0.001 | 1.04 (1.02 - 1.07) |
| MTV value | 0.94 | 0.97 | 0.80 | 0.01 | 1.005 (1.001 - 1.009) |
| TLG value | 0.95 | N/A | 0.99 | 0.047 | 0.999 (0.999 - 1.000) |
Abbreviations: HR, hazard ratio; CI, confidence interval; MTV, primary tumor metabolic tumor volume; TLG, primary tumor total lesion glycolysis.
5. Discussion
SUVmax is the most frequently evaluated PET/CT parameter in patients with lung cancer. In this study, as in previous studies (13-15), neither SUVmax nor SUVmean was associated with tumor behavior (lymphatic or distant metastasis) or patient survival. Some studies have also reported no association between survival of NSCLC patients and MTV or TLG values (21). Our study showed that while MTV and TLG were not associated with tumor behavior, they might play a role in predicting the survival of NSCLC patients, as shown in some other studies (22-24). In patients with SCC and those with lymph node metastasis, no associations between PET variables and survival could be established. By contrast, in ACA patients, PET/CT-derived parameters of the primary tumor were associated with survival. Specifically, in patients with ACA or lymph node or distant metastasis, higher MTV and lower TLG values were associated with shorter survival, and vice versa.
Predicting survival in lung cancer patients is challenging, as many factors are likely to influence survival. The relationship between MTV and TLG values and survival in patients with ACA or lymph node or distant metastasis, and why neither parameter is relevant for other NSCLC patients, remains to be elucidated. Although the risk of death from lung cancer would be expected to increase as the volume of the tumor increases, survival in patients with early-stage tumors is significantly better regardless of tumor volume. Whether a volumetric parameter is a predictor of survival in these patients remains to be addressed in studies with long-term follow-up. Moreover, given the highly effective treatment modalities for early-stage disease, it will be difficult to distinguish the predictive ability of a volumetric variable.
In our study, MTV was not associated with survival, but this may have been due to the small number of patients with early-stage disease, the effective treatment of these patients, and the short follow-up period. For patients with lymphatic and distant metastases, however, tumor volume has almost no effect on survival. Tumor volume can be easily determined using CT, but in patients with lymphatic or distant metastases, tumor volume does not change the stage –and survival– of the patients. Nonetheless, among our patients with advanced-TNM-stage NSCLC, survival decreased as MTV increased. This result suggests that the addition of metabolic information to volumetric information on the primary tumor could improve predictions of patient survival, even in patients with lymph node or distant metastasis.
Among NSCLC patients, metastasis occurs later in those with SCC, despite the rapid growth of these tumors, than in patients with ACA (25). In SCC tumors, volume may not indicate the aggressiveness of the tumor, unlike an increase in the volume of ACA tumors. Thus, for patients with ACA type NSCLC, a high MTV may be associated with worse survival.
Although TLG includes metabolic as well as volumetric information, its utility in predicting the survival of lung cancer patients is unclear. Our study suggests that determining TLG can provide information on survival but only in patients with ACA or distant metastasis.
One of the variables affecting the TLG value is the MTV value, and there is a good correlation between them. The opposite effects of MTV and TLG on patient survival in this study are therefore difficult to interpret. Survival was found to worsen as MTV levels increased and TLG levels decreased. The inverse relationship between these two parameters suggests a role for SUVmean, the other variable in the TLG calculation. Thus, a decrease in the TLG level with an increasing MTV implies a lower SUVmean. However, the association between the SUV mean value alone and survival was not significant. This suggests that SUVmean is heterogeneously associated with survival. According to our study, if there is no increase in the SUV mean as the MTV increases, survival will be worse. It may therefore be the case that the systemic effects of tumor cells are more important than their local activity, with negative consequences for survival. For patients with a primary tumor characterized by a high SUVmean and a small MTV, the higher local activity and fewer systemic effects of the tumor may improve survival.
This study had several limitations. First, the ROI in the calculation of MTV and TLG during FDG-PET examination was determined manually, which may lead to differences in the results depending on the operator. Second, both the retrospective nature of the study and the small size of the study population may have led to erroneous results. Similarly, the small number of patients with early-stage NSCLC reduced the accuracy of the analysis. A prospective study with more patients is needed to verify our results.
In conclusion, among the PET/CT-derived parameters, MTV and TLG can be used to predict the survival of patients with NSCLC. Specifically, in patients with ACA or lymphatic or distant metastasis, a higher MTV and lower TLG may be associated with shorter survival.