1. Background
Glomus jugulare tumors are a collection of paraganglia nerve cells located in the adventitia wall of the jugular bulb. Arising within the jugular foramen, they are localized to the jugular fossa in the temporal bone. These tumors are the second most common tumor inside the petrous bone and among the most common middle ear tumors. Many patients don’t need treatment. For those who do, the standard method is surgical resection. However, due to their vascular nature, a high bleeding tendency during surgery is a major concern, and surgical treatment of these tumors has a high mortality rate (1-3). They most commonly occur between the ages of 50 and 60, although they can affect people of any age group, with more cases reported in women than in men (4, 5). The origin of these tumors is usually from the main cells of the paraganglia located in the wall (endotentia) of the jugular bulb, and they are often treated in the advanced stages of diagnosis (6-8).
Despite being benign, these tumors can cause significant symptoms such as hearing loss, tinnitus, dizziness, and difficulty swallowing due to their proximity to the main blood vessels and nerves of the head and neck. Neurosurgeons often find it challenging to excise Glomus jugulare tumors due to their highly vascular nature and relatively inaccessible anatomical location. Consequently, excisions should be performed only if the patient is experiencing intracranial hypertension or compression of the brainstem. Intra-arterial embolization blocks the blood flow to the tumor, preventing excessive bleeding during surgery and facilitating the complete surgical removal of the lesion. Although embolization alone does not prevent tumor progression, it should be considered a preoperative surgical adjunct that can reduce bleeding during resection and provide anatomical details during surgery (9-12).
Glomus jugulare embolization is considered in different clinical settings as either a sole treatment or part of the treatment plan. These different clinical instances are as follows: The first clinical situation involves patients who are candidates for surgical excision of the tumor. In this case, embolization is performed to reduce the tumoral vascular blush, consequently decreasing the amount of bleeding during surgery and making the surgery easier. The second situation is when complete embolization is performed as the sole treatment for patients with small Glomus jugulare tumors who have severe cardiac problems or other baseline medical conditions that contraindicate safe skull base open surgery and general anesthesia. The third indication is partial embolization (as much as possible) of the lesion in patients with severe baseline medical conditions that contraindicate surgery and general anesthesia. On the other hand, embolization may increase the risk of arterial rupture (8, 13-22). Therefore, examining the results of preoperative embolization for the treatment of Glomus jugulare tumors is crucial, and there is a need to conduct studies to determine the efficacy, safety, and technical results of this procedure.
2. Objectives
The main objective of this study was to investigate the safety and technical success of preoperative intra-arterial embolization in the treatment of a group of patients with Glomus jugulare tumors. Additionally, we aimed to evaluate the effect of the procedure on the reduction of vascular blush diameter in these lesions.
3. Patients and Methods
This retrospective cohort study included 61 patients with Glomus jugulare tumors. Before surgery, all patients underwent magnetic resonance angiography (MRA) to evaluate tumor size and blood supply. Clinical symptoms, past medical histories, treatment histories, and data on short-term follow-up until surgery were gathered. This study received approval from the institutional review board and the ethics committee of the hospital (ethical approval code: IR.TUMS.IKHC.REC.1400.471).
All patients provided written informed consent before enrollment in the study. Patients of both genders with Glomus jugulare tumors were considered eligible. All patients were candidates for surgery and were referred to an interventional radiologist by an ENT surgeon to undergo intra-arterial embolization of the lesion before surgical excision (from 2016 to 2017). Participants were excluded if they were unwilling to participate in the study or were sensitive to angiography contrast material.
All embolization procedures were performed in one session. After local anesthesia in the groin, arterial access was made in the femoral area, the catheter was placed in the femoral artery, and advanced toward the common carotid artery. After cannulation of the carotid artery, common carotid angiography was performed and directed toward the location of the Glomus jugulare tumor. Then, selective angiography of the internal and external carotid arteries on the involved side was conducted. External carotid artery angiography, especially of the posterior auricular and ascending pharyngeal arteries, was primarily performed. Other possible branches that might feed the lesion were also assessed. Embolic agents were administered through the catheter to block blood flow to the specific tumor to shrink it. In all cases, embolization was performed using polyvinyl alcohol (PVA), which was diluted and slowly injected into the mentioned external carotid artery branches or other feeding arteries. Sometimes, glue, gelfoam, or lipiodol alone was used to embolize the tumor.
During the procedure, the diameters of the lesion in coronal, supero-inferior sagittal (SI sagittal), and antero-posterior sagittal (AP sagittal) views were measured before and immediately after embolization by an interventionist. Additionally, the percentage of devascularization was assessed subjectively. Major complications, such as bleeding, vascular injuries, and cranial nerve injuries, were monitored from the procedure until the day of surgery.
Statistical analyses were performed using SPSS version 28 (IBM corp. released 2021. IBM SPSS statistics for Windows, version 28.0. Armonk, NY: IBM corp). For continuous variables, the mean and standard deviation were calculated. The normal distribution of these variables was assessed by the Kolmogorov-Smirnov test. Categorical variables are presented as numbers and percentages. The comparison of continuous variables before and after the procedure was conducted using a paired t-test. Comparisons of these variables between different subgroups were performed using a t-test. Correlation of continuous variables was assessed using the Pearson correlation coefficient. All P-values lower than 0.05 were considered statistically significant.
4. Results
A total of 61 patients were included in this study. The mean age of the patients was 45.7 ± 14.1 years (range 12 - 69). Thirty-seven patients were female (60.7%) and 24 were male (39.3%). The most common symptoms were tinnitus, seen in 34 patients (55.7%), followed by hearing loss in 24 patients (39.3%) (Table 1).
| Symptom/Sign | No. (%) |
|---|---|
| Tinnitus | 34 (55.7) |
| Otitis | 2 (3.3) |
| Dizziness | 1 (1.6) |
| Hearing loss | 4 (39.3) |
| Headache | 7 (11.5) |
| Otalgia | 6 (9.8) |
| Hoarseness | 1 (1.6) |
| Ear hemorrhage | 1 (1.6) |
| Bell's palsy | 1 (1.6) |
None of the patients had a positive family history. Eight patients (13.1%) had undergone previous surgery. Ten patients (16.4%) had a history of previous angiography, with one being purely diagnostic and the other nine treated with embolization (four of which involved PVA). All angiographies were performed via the femoral puncture approach.
The feeding arteries included the external carotid arteries, carotid artery branches, and vertebral arteries. Specifically, the right and left external carotid arteries served as feeding arteries in 17 (27.9%) and 18 (29.5%) patients, respectively. Branches of the right and left carotid arteries were feeding arteries in 9 (14.8%) and 10 (16.4%) patients, respectively. The most common feeding arteries were the ascending pharyngeal arteries and posterior occipital arteries, observed in 25 (41%) and 17 (27.9%) patients, respectively. Some patients had more than one feeding artery (Table 2).
| Feeding Artery | No. (%) |
|---|---|
| Right external carotid artery | 17 (27.9) |
| Left external carotid artery | 18 (29.5) |
| Branches of the right external carotid artery | 9 (14.8) |
| Branches of Left external carotid artery | 10 (16.4) |
| Right vertebral artery | 1 (1.6) |
| Left vertebral artery | 2 (3.3) |
| Posterior occipital artery | 17 (3.3) |
| Auricular artery | 14 (3.3) |
| Superficial temporal artery | 3 (3.3) |
| Internal maxillary artery | 9 (3.3) |
| Ascending pharyngeal artery | 25 (3.3) |
| Facial artery | 1 (1.6) |
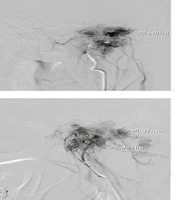
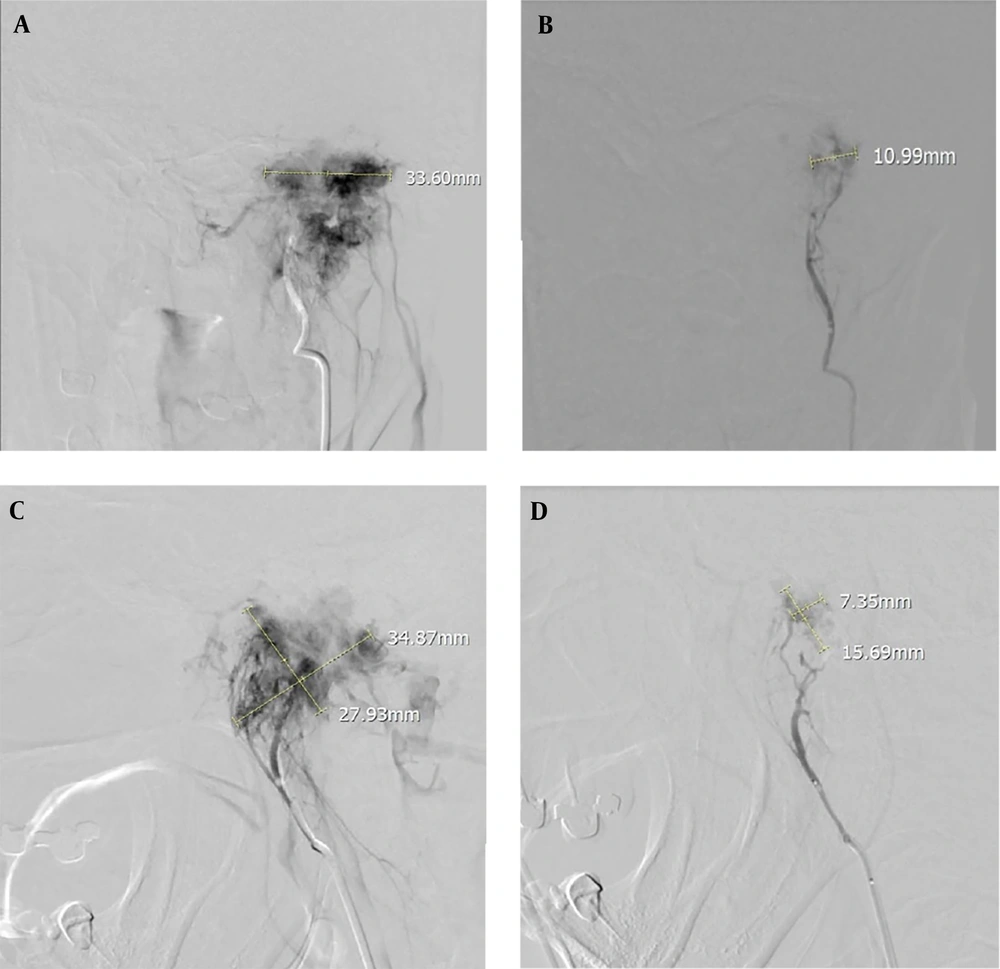
For the 35 patients with data on residual blush, no remnant vascular blush was seen in 6 patients (17.1%), while 29 patients (82.9%) had remnant vascular blush. Of these, the blush was mild in 21 (72.4%) patients, moderate in seven (24.1%), and considerable in one (3.4%) (Figure 1).
Anteroposterior and lateral DSA angiography of a 50-year-old woman with a left Glomus tumor supplied predominantly by the external carotid artery (ascending pharyngeal artery). A and C, Pre-embolization angiographic view; B and D, Angiographic view after embolization with polyvinyl alcohol (PVA) of the same case showing tumor devascularization with selective catheterization. Embolization shows a significant reduction in Glomus blood supply.
Pain following embolization was reported in 11 patients (18%). There were no other reported side effects or complications. All procedures were successfully completed without major technical issues. Surgery was planned within three days post-embolization for 24 patients (39.3%).
The mean pre-procedure coronal diameter was 29.1 ± 13.3 mm, which decreased to 16.8 ± 7.1 mm after embolization (P < 0.001). The corresponding measurements in the AP sagittal plane were 32.8 ± 14.4 mm pre-procedure and 19.3 ± 6.8 mm post-procedure (P < 0.001); in the SI sagittal plane, the measurements were 25.7 ± 13.4 mm pre-procedure and 14.1 ± 4.7 mm post-procedure (P < 0.001).
The mean percent of vascular blush size reduction for the coronal, AP sagittal, and SI sagittal planes were 39.04% ± 16.83, 37.43% ± 16.06, and 35.63% ± 24.41, respectively. There was a weak negative correlation between age and the percent of blush diameter reduction in the coronal view (r = - 0.357, P = 0.048).
The correlation coefficient of primary diameter with percent blush size reduction in the coronal, AP sagittal, and SI sagittal views were 0.42, 0.56, and 0.78, respectively (All P-values < 0.001).
In females, the mean percent blush size reduction in the coronal, AP sagittal, and SI sagittal views were 34.14% ± 17.34, 37.25% ± 14.72, and 33.07% ± 25.87, respectively, while in males, these figures were 46.22% ± 13.69, 37.73% ± 18.74, and 40.04% ± 21.88, respectively. The difference was statistically significant only in the coronal view (P = 0.04). The mean percent diameter reduction did not show a statistically significant difference between tumors with single-feeding arteries versus tumors with multiple-feeding arteries (All Ps > 0.15).
The total surgery time was 4.15 ± 0.76 hours, and the mean serum volume received during surgery was 1.87 ± 1.01 liters.
In total, only 4 patients needed to receive packed cells. In these patients, the mean percent blush size diameter reduction in the coronal, sagittal, and axial views were 40.20% ± 1.14, 47.72% ± 17.86, and 48.86% ± 16.49, respectively (P < 0.05).
5. Discussion
Glomus jugulare tumors are hypervascular tumors located in or near the temporal bone. They are initially slow-growing and asymptomatic but will eventually grow and invade the neck and surrounding structures. The tumor is more common in women in the fifth to sixth decades of life, though Glomus tumors can develop at any age. The higher prevalence in women is not precisely determined, but hormonal factors may play a role. Hormonal changes during pregnancy and post-menopause could contribute to this higher prevalence. Environmental factors, such as exposure to X-rays, chemicals, and radioactive materials, may also contribute to the increased prevalence in women. Additionally, genetic factors are relevant, as studies have shown a higher prevalence of Glomus tumors in certain families and individuals with a positive family history.
This retrospective cohort study included 61 patients. The findings demonstrated that preoperative embolization with PVA is an effective method to reduce vascular blush size before surgery. There were no considerable complications after the procedure, and pain after embolization was reported in only 18% of patients, indicating that the procedure is safe.
As mentioned, these tumors grow and invade adjacent structures, necessitating resection. Due to their hypervascular nature, patients are at risk of dangerous bleeding during surgery. Pre-surgical measures, such as intra-arterial embolization and radiotherapy, are employed to reduce bleeding during resection. During embolization, injecting embolizing agents (such as PVA, glue, and gelfoam) into feeding arteries decreases blood flow through the lesion. This reduces the risk of intraoperative bleeding and other complications, shortens operation time, and increases the surgeon’s control over the procedure. Improved control allows the surgeon to excise the lesion more completely, reducing the risk of recurrence (8, 16, 23-28).
We demonstrated a statistically significant reduction in vascular blush size among patients, which could suggest a lower bleeding tendency during surgery. However, confirming this assumption requires exact intraoperative bleeding volume measurement, which was unfortunately not performed in the current study. Additionally, the negative correlation between age and the mean percentage of vascular blush size reduction could be a warning for surgeons to pay closer attention to intraoperative bleeding tendencies in older patients, despite preoperative embolization.
The positive and clinically significant correlation between primary size and the percentage of blush size reduction is an encouraging finding for surgeons to consider embolization before surgery, even for large lesions.
Similar previous studies have shown consistent results. Helal et al. studied 29 patients with a mean tumor size of 3.4 cm. They reported at least a 50% reduction in tumor blush in 86.2% of patients, with none experiencing cranial nerve neuropathy. Near-total or total resection during surgery was achieved in 44.8% of patients. The average intraoperative blood loss was 888 cc, and 31% of patients required blood product transfusion during surgery (29).
In another similar study, no patients experienced severe complications after embolization and surgery. Additionally, tumor blush completely disappeared after embolization in 83.3% of patients. In this series, 69.2% of patients showed histological evidence of tumor necrosis after resection (16).
In summary, the results of this study indicate that embolization of Glomus jugulare tumors is a safe and effective method.
Our study has several limitations. First, the study design is a retrospective cohort, which typically lacks a control group. This limits the ability to establish causal relationships or compare the outcomes of embolization with alternative treatments or non-interventional approaches. Second, some patients were not candidates for surgery, and embolization was considered the main treatment for them. In these cases, the results of long-term embolization would be important. Our study lacks such information as all patients were candidates for immediate surgery. Potentially useful long-term outcome information, such as tumor recurrence rates and the durability and effectiveness of embolization over the long term, could not be determined in our study. Third, the study included a relatively small sample size of 61 patients. The findings may not be representative of the broader population of patients with Glomus jugulare tumors, and the generalizability of the results to other settings or populations may be limited. A fourth limitation is the lack of exact bleeding volume measurement during surgery. Additionally, we did not assess the ease of surgery by the surgeons, which is important. Finally, the lack of access to immediate and long-term post-surgery follow-up data (such as hemoglobin levels, recurrence rates, and other clinical parameters) that can determine the success of the final results is another limitation of the current study.
In conclusion, the findings of the current study suggest that intra-arterial embolization for Glomus jugular tumors could be safe and technically successful. The reduction in vascular blush size suggests that the procedure could effectively reduce bleeding during immediate surgery after embolization. However, caution is warranted due to the inherent limitations of the study, including its retrospective design, lack of long-term follow-up, and absence of a control group for comparison. Future studies employing prospective designs with long-term follow-up and including control groups are needed to validate these findings and provide more robust evidence. These efforts will contribute to further elucidating the effectiveness and safety of intra-arterial embolization in the management of Glomus jugular tumors.