1. Background
Malignant biliary obstruction (MBO) is a pathological blockage caused by malignancies in the bile duct, gallbladder, pancreas, ampulla of Vater, liver, etc. (1). Cholestasis in the bile duct can lead to the penetration of bile into the bloodstream. To avoid the adverse effects of hyperbilirubinemia, biliary drainage is necessary (2). A meta-analysis based on 26 articles revealed that preoperative biliary drainage improves the outcomes of malignant obstructive jaundice after surgery (3). For patients unable to undergo surgery, percutaneous transhepatic biliary drainage (PTBD) and endoscopic biliary drainage are commonly used strategies (4).
Percutaneous transhepatic biliary stenting (PTBS) is a supplement to PTBD, which can dredge the biliary tract, normalize plasma levels of bilirubin, and relieve MBO-associated symptoms (5). In PTBS, metal stents are superior to plastic stents due to longer patency and lower re-intervention rates (6). However, the growth of tumor cells and mucosal hyperplasia significantly limit the cumulative patency rate of metal stents, leading to the recurrence of biliary restenosis or obstruction (7). Encouragingly, the development of 125I particle stents opens new horizons. The implantation of 125I particle stents can not only dredge obstruction but also inhibit the growth of tumor cells in the bile duct (8). Pang et al. found that PTBS combined with 125I particles is more effective in inhibiting biliary re-obstruction and prolonging survival time than PTBS alone (9). Hasimu et al. also demonstrated that PTBS with 125I particles improves stent patency and extends survival time in patients with MBO (10). With the ability to prevent restenosis, 125I particle stent implantation shows great potential in the treatment of MBO.
With the widespread application of PTBS, increasing attention has been paid to complications. Early biliary infection (EBI) is a common early postoperative complication after PTBS, manifesting as shivering, fever, and even septic shock, posing a serious threat to patients' health and life (11). A previous study reported that 125I particle stent implantation is superior to conventional stents in reducing the risk of EBI, possibly due to the antimicrobial properties of integrated 125I and variations in stent composition, design, or material (12). Therefore, the prevention of EBI following 125I particle stent implantation is urgently needed.
Controlling risk factors is an effective strategy for preventing potential complications. Liu et al. revealed that diabetes, previous surgical or endoscopic treatment, procedure time > 60 min, and intraprocedural biliary hemorrhage are risk factors for EBI after PTBS (13). However, the risk factors of EBI following 125I particle stent implantation are not fully revealed. In this study, a total of 178 patients with MBO who underwent 125I particle stent implantation were enrolled. The baseline characteristics and perioperative parameters of these patients with or without EBI were retrospectively analyzed.
2. Objectives
This study aims to reveal potential risk factors of EBI after 125I particle stent implantation, providing guidance for the clinical treatment of MBO.
3. Patients and Methods
3.1. Subjects
This study received approval from the Ethics Committee of our hospital (ethical approval code: 2020-13). Informed consent was obtained from all cases.
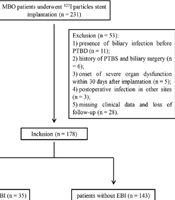
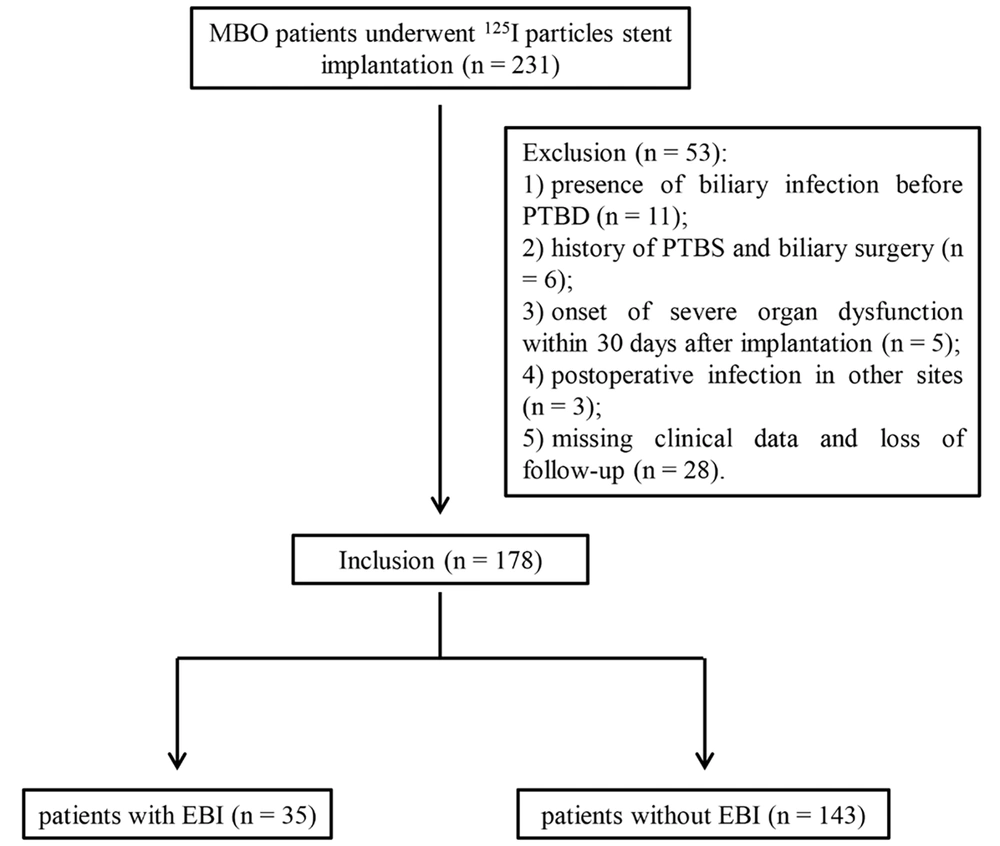
As a retrospective study, a total of 231 patients who underwent 125I particle stent implantation were collected from our hospital between January 2014 and December 2020. All patients were diagnosed with MBO based on pathological and imaging examinations. The inclusion criteria included: (1) > 18 years old; (2) unable or unwilling to undergo surgical operation; (3) first-time stent implantation. The exclusion criteria included: (1) benign biliary obstruction; (2) presence of biliary infection before PTBD (n = 11); (3) history of PTBS and biliary surgery (n = 6); (4) onset of severe organ dysfunction within 30 days after implantation (n = 5); (5) postoperative infection in other sites (n = 3); (6) missing clinical data and follow-up information (n = 28). Finally, a total of 178 patients (115 males and 63 females, aged 32 - 83 years) were included and analyzed in this study, comprising 35 patients with EBI and 143 patients without EBI (Figure 1). Patients included in the study underwent routine preoperative comprehensive assessments, including blood cell analysis, coagulation function tests, electrocardiography, liver and kidney function assessments, electrolyte analysis, abdominal computed tomography, magnetic resonance imaging, and magnetic resonance cholangiopancreatography. The baseline data, including sex, age, liver function [total bilirubin (TBIL), direct bilirubin (DBIL), aspartate aminotransferase (AST), alanine aminotransferase (ALT), and albumin (ALB)], blood routine [white blood cells (WBC), hemoglobin (HGB), and platelets (PLT)], coagulation function [prothrombin time (PT) and activated partial thromboplastin time (APTT)], comorbidity [diabetes mellitus (DM) and gallstones], obstruction location, and tumor types were collected.
3.2. 125I Particles Stent Implantation
The biliary 125I particle stent was purchased from Nanjing Micro-Tech Co., Ltd. (Nanjing, China), with a diameter of 10 mm (Appendix 1). Before stent implantation, PTBD was performed to determine the location and length of the obstruction in the bile duct under the digital subtraction angiography suite (Appendix 2). A guide wire was introduced through the PTBD tube, and the outer sheath was replaced. Using a single-curved catheter alongside the guide wire, the catheter traversed the narrowed bile duct segment. After removing the guide wire, cholangiography through the catheter precisely located and determined the length of the obstructed segment (Appendix 2). A 260 cm super-stiff, extra-long guide wire was then exchanged before withdrawing the catheter (Appendix 2). The number of 125I particles (diameter = 0. 8mm, length = 4.5 mm, effective radiation radius = 17 mm, half-life = 59.43 days; Beike Biotechnology, Beijing, China) was calculated by the radiotherapy treatment planning system (HGGR-2000; Hokai Medical Instruments, Zhuhai, China) according to the tumor status. The 125I particles are disinfected using 75% alcohol or high-temperature/high-pressure methods. They are then secured onto the particle stent delivery device using forceps. While loading 125I particles, avoid withdrawing the central metal support rod of the delivery device assembly. The biliary 125I particle stent is guided to the lesion site along a super-stiff guidewire and released upon confirming precise positioning using the proximal method (Appendix 2). After withdrawing the 125I particle stent delivery system, a standard biliary stent is advanced along the super-stiff guidewire to the site of bile duct obstruction (Appendix 2). The regular biliary stent is positioned to overlap with the radioactive particle stent, ensuring approximately a 10 mm extension beyond the lesion's upper and lower margins. Contrast imaging is then performed to confirm the release position and deployment status of the stent. Finally, the PTBD external drainage catheter (Cook Medical, USA) is to be retained in place and secured with appropriate dressing (Appendix 2).
Both PTBD and 125I particle stent implantation were performed by professional senior physicians. After the implantation for 2 - 3 weeks, unobstructed bile drainage was identified by cholangiography. Within 30 days after implantation, EBI was identified by the characteristics of fever (≥ 37.3ºC), persistent increase in white blood cells and neutrophils, positive bile culture, pain around the liver region, and/or continuous progression of jaundice. EBI within the initial 30 days post-stent implantation is consistent with clinical norms, emphasizing the critical early period when infectious complications are most prevalent.
For patients who develop EBI after 125I particle stent placement, if the PTBD drainage tube is not removed, biliary drainage can be performed through the PTBD drainage tube to control EBI. In cases of EBI, early administration of antibiotics is recommended. In our hospital, broad-spectrum antibiotics such as cefmetazole, cefoperazone-sulbactam, cefotiam, and piperacillin-tazobactam are used based on clinical experience until the results of drug sensitivity tests are available. Following the availability of sensitivity results, antibiotic therapy is adjusted accordingly. In cases where infection control is inadequate, and there is a tendency for worsening EBI, potent antibiotics like meropenem are employed. In this study, among 35 patients with EBI, appropriate anti-inflammatory treatment effectively controlled biliary infections.
3.3. Statistical Analysis
Statistical analysis was performed using SPSS 25.0 (IBM Corp. Released 2017. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp.). Continuous data underwent normality assessment, and for normally distributed metric data, the independent t-test was applied, with results presented as mean ± standard deviation. Categorical data were presented as number (percentage), and those between two groups were compared by χ2 test. This study classifies risk factors associated with EBI into two categories: Patient-related and surgical-related factors. Patient-related factors encompass sex, age, gallstones in the gallbladder or bile ducts, types of malignant tumor, obstruction location (low- or high-level), comorbidities (such as diabetes), preoperative liver function, blood routine, PT, APTT, and postoperative intrahepatic pneumobilia. Surgical factors include 125I particle numbers, intraoperative biliary bleeding, PTBD, time from PTBD to 125I stent implantation (days), time from jaundice to 125I stent implantation (days), and drainage tube removal. Observations are independent of each other. Univariate and multivariable logistic regression analyses were employed to identify independent risk factors influencing postoperative EBI, with those having P < 0.05 subsequently subjected to multivariate logistic regression analysis to identify independent risk factors for EBI after 125I particle stent placement. P-values less than 0.05 were considered statistically significant.
4. Results
4.1. Distribution of Bacterial Species in Early Biliary Infection
Within 30 days after implantation, EBI was observed in 35 patients (19.7%). The bacterial species in these patients were identified by bile culture, with positive results in all 35 cases. Escherichia coli (N = 17) was the dominant gram-negative bacterium in bile samples, followed by Klebsiella pneumoniae (N = 5), Pseudomonas putida (N = 3), and Pseudomonas aeruginosa (N = 1). Gram-positive bacteria included Enterococcus faecalis (N = 4) and Staphylococcus (N = 1). Additionally, mixed bacterial infections and Candida were identified in 3 and 1 cases, respectively.
4.2. Comparisons of Baseline Characteristics in Patients with and Without Early Biliary Infection
The baseline characteristics of patients with and without EBI were compared. As listed in Table 1, there were no significant differences in sex, age, liver function (TBIL, DBIL, AST, ALT, and ALB), blood routine (WBC, HGB, and PLT), coagulation function (PT and APTT), and tumor types between the non-EBI and EBI groups. Notably, diabetes and gallstones (in the gallbladder or bile ducts) were more frequent in EBI cases compared to non-EBI cases (P < 0.01). Patients with high-level obstruction were also more likely to suffer from EBI (P = 0.013).
| Parameters | Non-EBI (n = 143) | EBI (n = 35) | P-value |
|---|---|---|---|
| Sex | 0.809 | ||
| Male | 93 (65.0) | 22 (62.9) | |
| Female | 50 (35.0) | 13 (37.1) | |
| Age (y) | 60.87 ± 10.75 | 61.51 ± 9.92 | 0.749 |
| Liver function | |||
| TBIL (μmol/L) | 342.82 ± 151.80 | 328.94 ± 140.95 | 0.624 |
| DBIL (μmol/L) | 274.36 ± 106.43 | 274.78 ± 116.25 | 0.984 |
| AST (IU/L) | 126.28 ± 118.43 | 172.20 ± 170.00 | 0.063 |
| ALT (IU/L) | 158.07 ± 148.84 | 178.86 ± 174.92 | 0.476 |
| ALB (g/L) | 37.03 ± 4.96 | 36.06 ± 6.56 | 0.330 |
| Blood routine | |||
| WBC (× 109/L) | 7.14 ± 1.86 | 7.26 ± 2.18 | 0.744 |
| HGB (g/L) | 127.28 ± 16.73 | 123.17 ± 17.19 | 0.197 |
| PLT (× 109/L) | 297.92 ± 97.87 | 298.60 ± 84.33 | 0.970 |
| Coagulation function | |||
| PT (s) | 12.82 ± 1.56 | 13.12 ± 1.37 | 0.290 |
| APTT (s) | 36.83 ± 4.59 | 37.13 ± 3.81 | 0.726 |
| Comorbidity | |||
| Diabetes | 12 (8.4) | 10 (28.6) | < 0.001 a |
| Gallstones in the gallbladder or bile ducts | 9 (6.3) | 8 (22.9) | 0.003 a |
| Obstruction location | 0.013 a | ||
| High-level obstruction | 35 (24.5) | 16 (45.7) | |
| Low-level obstruction | 108 (75.5) | 19 (54.3) | |
| Types of malignant tumor | 0.998 | ||
| Bile duct | 74 (51.7) | 18 (51.4) | |
| Pancreas | 34 (23.8) | 9 (25.8) | |
| Secondary | 16 (11.2) | 4 (11.4) | |
| Gallbladder | 9 (6.3) | 2 (5.7) | |
| Others | 10 (7.0) | 2 (5.7) |
Abbreviations: EBI, early biliary infection; TBIL, total bilirubin; DBIL, direct bilirubin; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALB, albumin; WBC, white blood cell; HGB, hemoglobin; PLT, platelet; PT, prothrombin time; APTT, activated partial thromboplastin time.
a Significantly different at P < 0.05.
4.3. Comparisons of Perioperative Parameters in Patients with and Without Early Biliary Infection
The perioperative parameters were subsequently compared. The time from PTBD to 125I stent implantation was significantly longer in non-EBI cases than in EBI cases (P = 0.009). However, the time from jaundice to 125I stent implantation and the number of particles were not significantly different between cases with and without EBI. Additionally, the removal of the drainage tube was not associated with the onset of EBI. Notably, intraoperative biliary bleeding and postoperative intrahepatic pneumobilia were more likely to be found in EBI cases compared to non-EBI cases (P < 0.01) (Table 2).
| Parameters | Non-EBI (n = 143) | EBI (n = 35) | P-value |
|---|---|---|---|
| Time from jaundice to 125I sent implantation (days) | 50.73 ± 31.03 | 45.49 ± 17.64 | 0.339 |
| Time from PTBD to 125I sent implantation (days) | 22.55 ± 25.13 | 14.80 ± 16.89 | 0.009 b |
| Particle numbers | 15.15 ± 4.62 | 15.26 ± 5.02 | 0.907 |
| Intraoperative biliary bleeding | 9 (6.3) | 8 (22.9) | 0.003 b |
| Drainage tube removal | 97 (67.8) | 22 (62.9) | 0.575 |
| Postoperative intrahepatic pneumobilia | 53 (37.1) | 22 (62.9) | 0.006 b |
Abbreviations: EBI, early biliary infection; PTBD, percutaneous transhepatic biliary drainage.
a Values are expressed as mean ± SD or No. (%).
b Significantly different at P < 0.05.
4.4. Identification of Risk Factors for Early Biliary Infection
Based on the parameters with significant differences, multivariate logistic regression analysis was performed to determine the potential risk factors for EBI. As shown in Table 3, diabetes, gallstones in the gallbladder or bile ducts, high-level obstruction, intraoperative biliary bleeding, and postoperative intrahepatic pneumobilia were all identified as risk factors for EBI in patients who underwent 125I particle stent implantation (P < 0.05). Conversely, the time from PTBD to 125I stent implantation was identified as a protective factor against EBI (P = 0.012).
| Parameters | B-value | P-value a | OR | 95% CI |
|---|---|---|---|---|
| Diabetes | 1.203 | 0.029 | 3.329 | 1.129 - 9.819 |
| Gallstones in the gallbladder or bile ducts | 1.241 | 0.040 | 3.459 | 1.060 - 11.283 |
| High obstruction | 1.101 | 0.015 | 3.008 | 1.243 - 7.280 |
| Intraoperative biliary bleeding | 1.689 | 0.008 | 5.416 | 1.569 - 18.696 |
| Postoperative intrahepatic pneumobilia | 0.976 | 0.029 | 2.655 | 1.108 - 6.362 |
| Time from PTBD to 125I sent implantation | -0.035 | 0.012 | 0.966 | 0.940 - 0.992 |
Abbreviations: B, regression coefficient; OR, odds ratio; CI, confidence interval; PTBD, percutaneous transhepatic biliary drainage.
a Significantly different at P < 0.05.
5. Discussion
Malignant biliary obstruction is an ominous disorder of biliary obstruction and cholestasis caused by the invasion of malignant tumors (14). Malignant biliary obstruction is accompanied by high mortality mainly due to tumor progression, liver failure, and sepsis (15). Percutaneous transhepatic biliary stenting, characterized by minimal invasion and high efficiency, is a common therapeutic strategy for MBO. The development of 125I particle stents brings great advantages in prolonging biliary patency and survival time (13). However, the onset of complications, including EBI, seriously affects the quality of life. In this study, EBI was observed in 35 patients (19.7%) after 125I stent implantation. Bile culture determined that the pathogens of EBI were mainly gut-derived bacteria, such as E.coli, K.pneumoniae, and E.faecalis. Additionally, several risk factors for EBI were identified, including diabetes, gallstones in the gallbladder or bile ducts, high-level obstruction, short time from PTBD to 125I stent implantation, intraoperative biliary bleeding, and postoperative intrahepatic pneumobilia.
Diabetes is a common metabolic disease characterized by hyperglycemia (16). Many previous studies have identified diabetes as a risk factor for EBI after PTBS (11, 13). In this study, diabetes was more frequent in the EBI group than in the non-EBI group and was subsequently determined to be a risk factor for EBI after 125I particle stent implantation. Our findings are consistent with previous studies, illustrating that diabetes is a risk for the onset of EBI following 125I particle stent implantation. The possible mechanisms include: (1) the reduction of insulin with anti-inflammatory effects (17); (2) weakened immune defense, leading to microbial invasion and infection (18); (3) diabetes-associated vascular disorders affecting blood flow around the bile duct, benefiting the growth of anaerobic bacteria under a hypoxic environment (19); and (4) hyperglycemia benefiting the growth of many bacteria in the bile duct (20). Therefore, preoperative control of blood glucose is urgently needed for patients requiring 125I particle stent implantation.
Gallstones grow inside the gallbladder or biliary tract, causing intermittent obstruction of the cystic duct (21). Gallstones can lead to a series of disorders, such as pain, jaundice, infection, and acute pancreatitis (22). A previous study reported that gallstones are a risk factor for cholecystitis within 30 days after PTBS (23). In this study, gallstones in the gallbladder or bile ducts were determined to be a risk factor for EBI after 125I particle stent implantation. This result demonstrates the adverse role of gallstones in the outcomes of 125I stent implantation. The underlying mechanisms may be that gallstones can block the stent with the contraction of the gallbladder and the flow of bile, and the obstruction-induced cholestasis benefits bacterial growth, contributing to EBI. To avoid the adverse effects of gallstones, premature removal of PTBD drainage tubes is not recommended after stent implantation. When gallstones block the stent, they can be removed through the drainage tubes.
The location of obstruction is closely associated with the risk of biliary infection after drainage. Zhou et al. reported that the location of obstruction is a risk factor for EBI following PTBS (24). Consistent with previous studies, this study also revealed that high-level obstruction is a risk factor for EBI after 125I particle stent implantation. High-level biliary obstruction is characterized by an insidious onset and special anatomical location, which may lead to an increased risk of stent implantation and predispose to a variety of complications, such as EBI (25). For patients with high-level obstruction, accurate location of the obstruction based on preoperative cholangiography is necessary, and an appropriate stent should be customized to achieve sufficient drainage and reduce the risk of EBI.
Intraoperative biliary bleeding may occur due to mechanical damage caused by punctures, wire guides, and stents (26). Biliary bleeding can increase the risk of biliary infection by bringing contaminated bile into the bloodstream. Liu et al. reported that intraoperative biliary bleeding is an independent risk factor for EBI after PTBS (13). Consistently, this study also revealed that intraoperative biliary bleeding is a risk factor for EBI after 125I particle stent implantation. Therefore, avoiding vascular damage during interventional therapy is particularly important for reducing the risk of EBI.
Under normal circumstances, no gas is present in the bile duct. In this study, postoperative intrahepatic pneumobilia was observed in 75 cases after 125I particle stent implantation, which was more frequent in the EBI group than in the non-EBI group. The subsequent analysis determined a positive correlation between postoperative intrahepatic pneumobilia and EBI. The respiration of some bacteria contributing to EBI may lead to the onset of intrahepatic pneumobilia. After identifying bacterial gas in patients with postoperative intrahepatic pneumobilia, the drainage tube should be temporarily retained for flushing bile ducts.
The time from PTBD to 125I implantation was further determined to be a protective factor for EBI. Patients with MBO usually have impaired immune function (27). Hyperbilirubinemia can further weaken cellular defense responses by stimulating cytotoxic reactions (28). Given that the normal protective barrier of the bile duct is easily damaged, interventional therapies, including PTBD, have a high risk of inducing biliary infection. Therefore, it is better to wait for the improvement of bilirubin, liver and coagulation function, and remission of edema in the bile duct wall before 125I particle stent implantation. Our findings indicate that an appropriate extension of the time from PTBD to 125I stent implantation is beneficial to reducing the risk of EBI.
In conclusion, patients with MBO have a relatively high incidence of EBI (19.7%) after 125I particle stent implantation. Diabetes, gallstones in the gallbladder or bile ducts, high-level obstruction, intraoperative biliary bleeding, and postoperative intrahepatic pneumobilia are risk factors for EBI. The time from PTBD to 125I stent implantation is a protective factor for EBI. However, this study is based on data from a single center with a limited sample size, potentially leading to biased results. The risk factors of EBI are not only limited to those elucidated in this study. Further research with a larger sample size and consideration of additional factors, such as the time of onset of malignancy, patient weight loss and malnutrition, and immunosuppression, is warranted.