1. Background
Liver cirrhosis is the most frequent cause of portal hypertension (1). In contrast, idiopathic portal hypertension (IPH) is a relatively rare clinical condition characterized by portal hypertension in the absence of cirrhosis. The etiology of IPH remains unclear and may be associated with factors such as immune disorders, bacterial infections, trace metal poisoning, drug therapy, impaired hepatic circulation, and thrombosis (2). Due to the low prevalence of IPH, clinicians are currently less familiar with its imaging features, some of which resemble those of cirrhosis, leading to the frequent misdiagnosis of IPH as cirrhosis in clinical practice. Liver biopsy serves as the gold standard for diagnosing IPH. Given that the pathological features of IPH and cirrhosis differ, their imaging manifestations may also vary. In this study, we conducted a retrospective analysis comparing the imaging features [computed tomography (CT), magnetic resonance imaging (MRI) , etc] of patients with pathologically confirmed IPH and cirrhosis with the aim of enhancing clinicians’ understanding of IPH and reducing the likelihood of misdiagnosis during initial evaluations.
2. Objectives
The aim of this study was to summarize and analyze the imaging, pathological, and serological features of idiopathic portal hypertension and cirrhosis to reduce misdiagnosis in clinical practice.
3. Patients and Methods
3.1. General Information and Patients’ Enrollment
We conducted a retrospective study on the clinical and imaging data of 14 patients with pathologically confirmed IPH who were admitted to the Affiliated Hospital of Hangzhou Normal University from January 2018 to January 2023. Clinical and imaging data from 30 patients with clinically and pathologically confirmed cirrhosis were collected as controls. The aim of this study was to compare, analyze, and summarize the imaging characteristics of IPH. The IPH group consisted of 5 male and 9 female patients, with a mean age of 54 ± 8.9 years. The etiology of 13 cases was unknown, and 1 case was considered to be caused by oxaliplatin. The cirrhosis group included 20 male and 10 female patients, with an average age of 49 ± 14.5 years, of whom 25 cases had viral hepatitis B, 3 had autoimmune liver disease, 1 had hepatomegaly, and 1 had familial hereditary cholestasis syndrome. Six patients with hepatocellular carcinoma were included in the cirrhosis group.
The inclusion and exclusion criteria for IPH were as follows (3):
- Presence of clinical signs of portal hypertension, including splenomegaly/hypersplenism, esophageal varices, non-neoplastic ascites, elevated hepatic venous pressure gradient, and formation of portal collateral circulation;
- Exclusion of liver cirrhosis through biopsy;
- Exclusion of chronic liver diseases that could lead to cirrhosis, such as chronic hepatitis B/C, nonalcoholic steatohepatitis/alcoholic steatohepatitis, autoimmune hepatitis, hereditary hemochromatosis, hepatolenticular degeneration, and primary biliary cholangitis;
- Exclusion of diseases that could cause nonsclerotic portal hypertension, such as congenital hepatic fibrosis, nodular regenerative hyperplasia, and schistosomiasis.
Pathologically, the diagnostic features of IPH are as follows (4):
- Stenosis or occlusion of small branches of the portal vein, along with thickening or fibrosis of the walls of large branches of the portal vein;
- Dilatation of small branches of the portal vein and their “herniation” into the surrounding liver parenchyma;
- Interstitial fibrosis of the hepatic portal area, with or without inflammation;
- Regeneration of nodular hyperplasia.
3.2. Imaging Information
We collected CT-enhanced and MR-enhanced imaging data from 14 patients with clinically and pathologically confirmed IPH and 30 patients with cirrhosis. The following manifestations were analyzed and compared: Spleen thickness and length, liver morphology, hepatic lobe atrophy, hyperplasia, portal vein thrombosis, arteriovenous phase liver perfusion, regenerative nodules, focal nodular hyperplasia-like lesions of the liver, portal vein morphology, splenorenal shunt, and hepatorenal shunt. The aim of this study was to investigate the correlation between imaging findings and pathological manifestations.
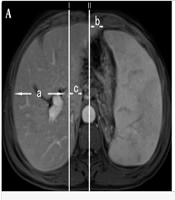
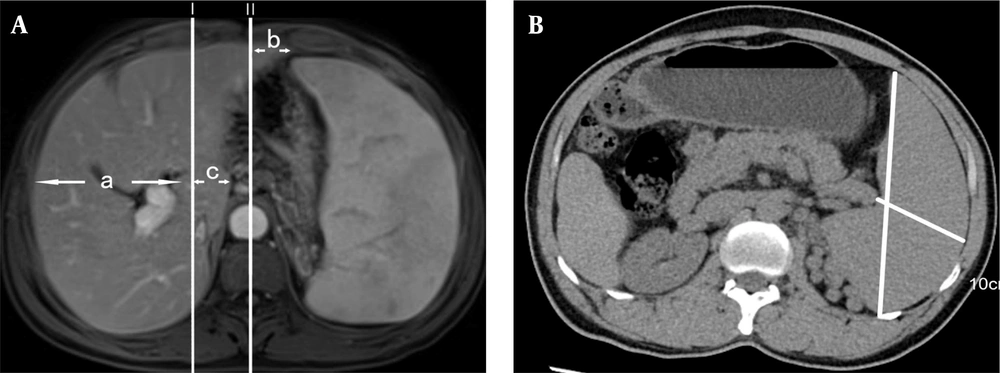
For hepatic lobe atrophy and hyperplasia, we followed the method proposed by TianRan et al. (5). We selected the intrahepatic bifurcation of the main trunk of the portal vein, which provides better visualization of the right and left lobes and is fixed in position. Two lines, Line I and Line II, were drawn. Line I represents the sagittal line tangent to the rightmost edge of the inferior vena cava, while Line II represents the median sagittal line. The measured indices were as follows: (a) the distance between the rightmost margin of the right lobe of the liver and Line I; (b) the distance from the leftmost margin of the left lobe of the liver to Line II; and (c) the distance from the leftmost margin of the caudate lobe to Line I (Figure 1A). Measurements of the spleen's thickness and length were taken at the splenic hilum, with the thickness being the shortest diameter from the inner edge of the spleen to the outer edge at the level of the center of the hilum, and the length being the longest diameter of the spleen at the level of the center of the hilum (straight line front and rear) (Figure 1B). All imaging data, including numerical measurements, were evaluated by two experienced senior imaging physicians, and the average of the data from both physicians was used.
3.3. Histopathology of the Liver
Liver biopsy specimens from the patients were embedded in paraffin, sectioned consecutively, stained with hematoxylin and eosin (H&E) and special stains, and subjected to immunohistochemistry. Microscopic interpretation was performed by two experienced pathologists.
3.4. Serological Examination
The patient's blood was drawn from a vein on an empty stomach the day before the biopsy. After centrifugation to separate serum, liver function indicators, including direct bilirubin (DBIL), indirect bilirubin (IBIL), total protein (TP), albumin (ALB), globulin (GLB), the albumin-globulin ratio (A/G ratio), alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), cholinesterase (CHE), γ-glutamyl transferase (GGT), and total bile acid (TBA), were measured using our hospital's fully automated biochemical analyzer (Hitachi 7180 Fully Automated Biochemical Analyzer). Routine blood counts, including white blood cell (WBC) counts, absolute neutrophil counts (ANC), red blood cell (RBC) counts, and blood platelet (PLT) counts, as well as coagulation indicators, including prothrombin time (PT), international normalized ratio (INR), activated partial thromboplastin time (APTT), thrombin time (TT), and fibrinogen (FG), were also assessed.
3.5. Statistical Methods
All the data were analyzed using SPSS version 26 (IBM Corp. Released 2019. IBM SPSS Statistics for Windows, Version 26.0. Armonk, NY: IBM Corp.). Continuous variables that conformed to a normal distribution are expressed as the mean ± standard deviation (SD), while continuous variables that did not conform to a normal distribution are expressed as the median (interquartile range) [M(IQR)]. Categorical variables are expressed as percentages (%). Comparisons between groups were accomplished using the t-test or the Mann-Whitney U test for measurement data and the chi-square test or Fisher's exact test for count data. Based on the measurements of hepatic lobe atrophy and hyperplasia, receiver operating characteristic (ROC) curves were plotted for the diagnosis of IPH, and the Youden index was applied to derive the optimal cut-off value. The following diagnostic indices were then calculated: Sensitivity, specificity, positive predictive value, and negative predictive value.
4. Results
4.1. Summary of Patients' Clinical Baseline Data
The clinical baseline data of the IPH patients and cirrhotic patients are detailed in Table 1. The mean age in the IPH group was 54 ± 8.9 years, including 5 male patients and 9 female patients. The mean age of patients in the cirrhosis group was 49 ± 14.5 years, with 20 male and 10 female patients, including one patient who underwent splenectomy. Regarding liver function indices, there were significant differences between the IPH group and the cirrhosis group in terms of serum ALB concentration, GLB concentration, A/G ratio, alanine transaminase (ALT), aspartate transaminase (AST), gamma-glutamyl transpeptidase (GGT), and total bile acid (TBA) levels (P < 0.05). There was a significant difference in the WBC count between the IPH group and the cirrhosis group (P < 0.05). In terms of coagulation function indices, there were significant differences in TT and FG between the IPH group and the cirrhosis group (P < 0.05).
| Parameter | Liver Cirrhosis (n = 30) | IPH (n = 14) | X2/t/Z | P-Value |
|---|---|---|---|---|
| Gender | 3.727 | 0.054 | ||
| Male | 20 (66.7) | 5 (35.7) | ||
| Female | 10 (33.3) | 9 (64.3) | ||
| Age | 49.47 ± 14.50 | 54.07 ± 8.88 | - 1.092 | 0.281 |
| DBIL (μmol/L) | 5.90 [4.03,7.70] | 4.35 [2.85,6.60] | - 1.55 | 0.121 |
| IBIL (μmol/L) | 14.90 [11.33,20.38] | 12.15 [7.88,25.92] | - 0.655 | 0.512 |
| TP (g/L) | 68.02 ± 7.40 | 63.71 ± 7.32 | 1.802 | 0.079 |
| ALB (g/L) | 38.29 ± 6.02 | 38.57 ± 5.41 | - 0.151 | 0.881 |
| GLB (g/L) | 29.73 ± 5.99 | 24.79 ± 3.72 | 2.836 | 0.007 |
| A/G Ratio | 1.34 ± 0.35 | 1.58 ± 0.28 | - 2.243 | 0.030 |
| ALT (U/L) | 32.50 [21.00,47.75] | 19.00 [17.00,25.75] | - 2.599 | 0.009 |
| AST (U/L) | 35.50 [24.50,44.25] | 24.00 [19.25,30.50] | - 1.992 | 0.046 |
| ALP (U/L) | 122.50 [101.50,147.75] | 98.50 [63.75,157.00] | - 1.222 | 0.222 |
| CHE | 5187.10 ± 2467.29 | 5039.71 ± 1280.86 | 0.261 | 0.796 |
| GGT (U/L) | 50.50 [35.25,112.50] | 31.00 [25.00,43.00] | - 2.521 | 0.012 |
| TBA (U/L) | 18.20 [7.20,53.27] | 6.55 [4.05,9.43] | - 2.797 | 0.005 |
| WBC (109 /L) | 4.30 [3.04,5.73] | 2.48 [2.06,3.45] | - 2.356 | 0.018 |
| ANC (109 /L) | 2.33 [1.94,3.70] | 1.83 [1.40,2.63] | - 1.399 | 0.162 |
| RBC (1012 /L) | 4.21 ± 0.85 | 3.69 ± 0.92 | 1.845 | 0.072 |
| PLT (s) | 80.50 [53.50,173.25] | 57.50 [38.25,125.00] | - 1.084 | 0.279 |
| PT (s) | 12.55 [11.85,13.70] | 12.55 [11.80,13.70] | - 0.088 | 0.930 |
| INR (s) | 1.19 [1.11,1.30] | 1.17 [1.11,1.27] | - 0.479 | 0.632 |
| APTT (s) | 30.30 [28.88,33.62] | 27.80 [26.13,32.75] | - 1.764 | 0.078 |
| TT (s) | 18.45 [17.40,19.48] | 16.25 [15.77,16.78] | - 4.335 | < 0.001 |
| FG (g/L) | 1.85 [1.57,2.37] | 2.40 [2.16,3.04] | - 2.848 | 0.004 |
4.2. Summary of IPH Imaging Features
4.2.1. Comparison of the Quantitative Values of Hepatic Lobe Atrophy and Hyperplasia and Spleen Thickness and Length Between the IPH Group and the Cirrhosis Group
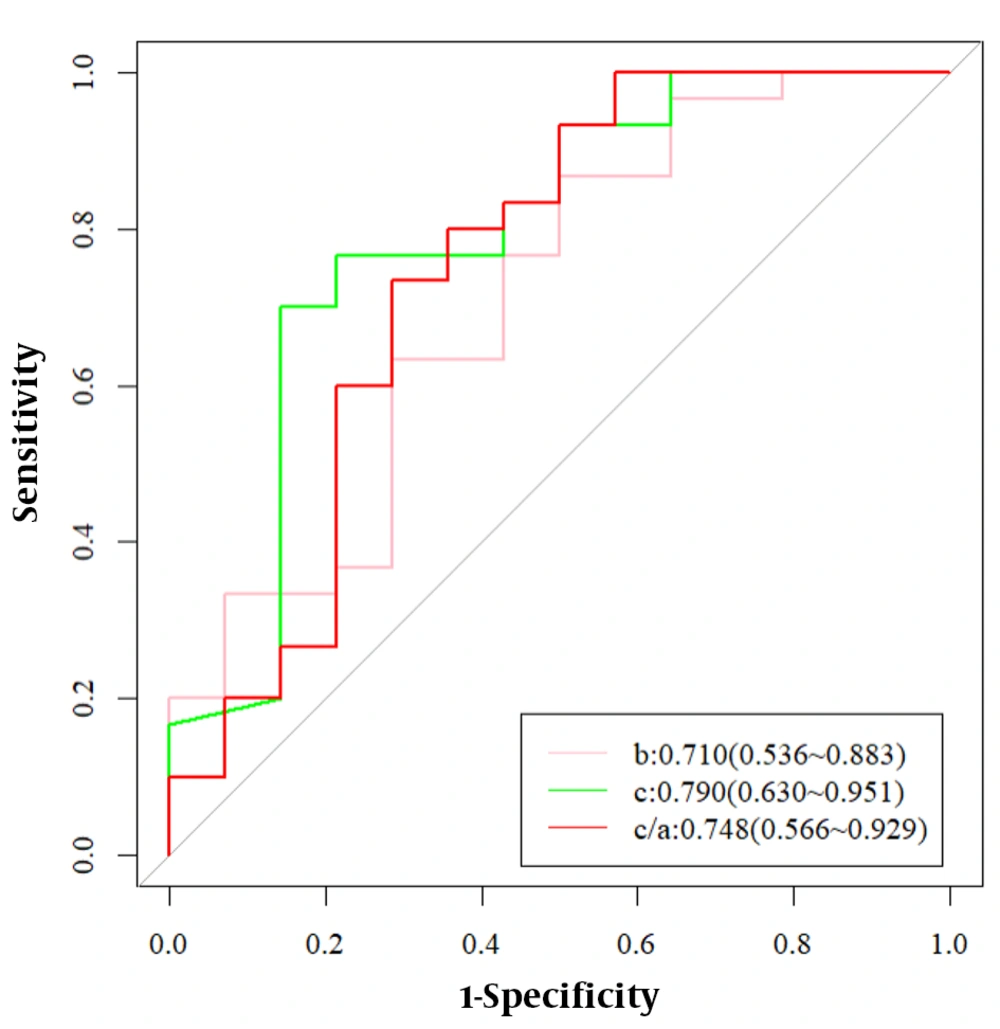
The median [IQR] spleen length was 15.75 cm [12.55, 17.15] in the IPH group, while it was 10.20 cm [9.20, 12.90] in the cirrhosis group (P = 0.001). The mean (SD) spleen thickness was 6.31 ± 1.56 cm in the IPH group, while it was 4.94 ± 0.95 cm in the cirrhosis group (P = 0.007) (Table 2). There was a significant difference in spleen thickness and length between the IPH group and the cirrhosis group (P < 0.05). The parameters b (coronal diameter of the left lobe of the liver), c (coronal diameter of the caudate lobe), and c/a (caudal right ratio) were significantly different between the IPH group and the cirrhosis group (P < 0.05) (Table 3). The diagnostic efficacy of hepatic lobe atrophy and hyperplasia for IPH is shown in Table 4.
| Parameter | Liver Cirrhosis (n = 30) | IPH (n = 14) | Z/X2 | P-Value |
|---|---|---|---|---|
| Abnormal liver morphology | 21 (70) | 14 (100) | 3.597 | 0.058 |
| Portal vein thrombosis | 1 (3.3) | 1 (7.1) | - | 0.540 b |
| Arterial and venous phase perfusion abnormalities | 3 (10) | 5 (35.7) | 2.69 | 0.101 |
| Liver regenerative nodules | 8 (26.7) | 0 (0) | 2.946 | 0.086 |
| Focal nodular Hyperplasia-like Lesions | 0 (0) | 2 (14.3) | - | 0.096 b |
| Abnormal portal vein morphology | 9 (30) | 14 (100) | 18.748 | < 0.001 |
| Spleen-kidney shunt | 4 (13.3) | 1 (7.1) | 0.009 | 0.926 |
| Gastro-renal shunt | 2 (6.7) | 3 (21.4) | 0.86 | 0.354 |
| Spleen length (cm) | 10.20 [9.20,12.90] | 15.75 [12.55,17.15] | - 3.384 | 0.001 |
| Spleen thickness (cm) | 4.94 ± 0.95 | 6.31 ± 1.56 | - 3.042 | 0.007 |
Comparison of Imaging Features Between Idiopathic Portal Hypertension and Cirrhosis Patients a
| Parameter | Liver Cirrhosis (n = 30) | IPH (n = 14) | t/Z | P-Value |
|---|---|---|---|---|
| Coronal diameter of the right lobe of the liver (mm) | 8.84 ± 0.98 | 8.91 ± 0.95 | -0.223 | 0.824 |
| Coronal diameter of the left lobe of the liver (mm) | 5.37 [4.04,7.96] | 3.32 [2.41,6.12] | -2.218 | 0.027 |
| Coronal diameter of the caudate lobe (mm) | 3.69 [3.34,3.80] | 2.90 [2.40,3.26] | -3.077 | 0.002 |
| Caudal right ratio | 0.42 [0.37, 0.44] | 0.31 [0.26, 0.39] | -2.642 | 0.009 |
Quantitative Evaluation of Liver Lobe Atrophy and Hyperplasia a
| Parameters | AUC | Cutoff | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|---|
| Coronal diameter of the left lobe of the liver | 0.710 | 3.205 | 0.867 | 0.500 | 0.788 | 0.267 |
| Coronal diameter of the caudate lobe | 0.790 | 3.440 | 0.700 | 0.857 | 0.913 | 0.350 |
| Caudal right ratio | 0.748 | 0.378 | 0.733 | 0.714 | 0.846 | 0.373 |
Diagnostic Efficacy of Hepatic Lobe Atrophy and Hyperplasia for Idiopathic Portal Hypertension
Receiver operating characteristic analysis of the b parameter (coronal diameter of the left lobe of the liver) for differentiation of IPH from cirrhosis patients showed an area under the curve (AUC) of 0.71; with a cutoff value of 3.205 cm, it showed a specificity of 0.500 and a sensitivity of 0.867. Receiver operating characteristicanalysis of the c parameter (coronal diameter of the caudate lobe) for differentiation of IPH from cirrhosis patients showed an AUC of 0.79; with a cutoff value of 3.440 cm, it showed a specificity of 0.857 and a sensitivity of 0.700. Receiver operating characteristicanalysis of the c/a parameter (caudal right ratio) for differentiation of IPH from cirrhosis patients showed an AUC of 0.748; with a cutoff value of 0.378, it showed a specificity of 0.714 and a sensitivity of 0.733. The ROC curves for atrophy and hyperplasia of the liver for the diagnosis of IPH are shown in Figure 2.
4.2.2. Changes in Liver Morphology and Liver Parenchyma in the IPH and Cirrhosis Groups
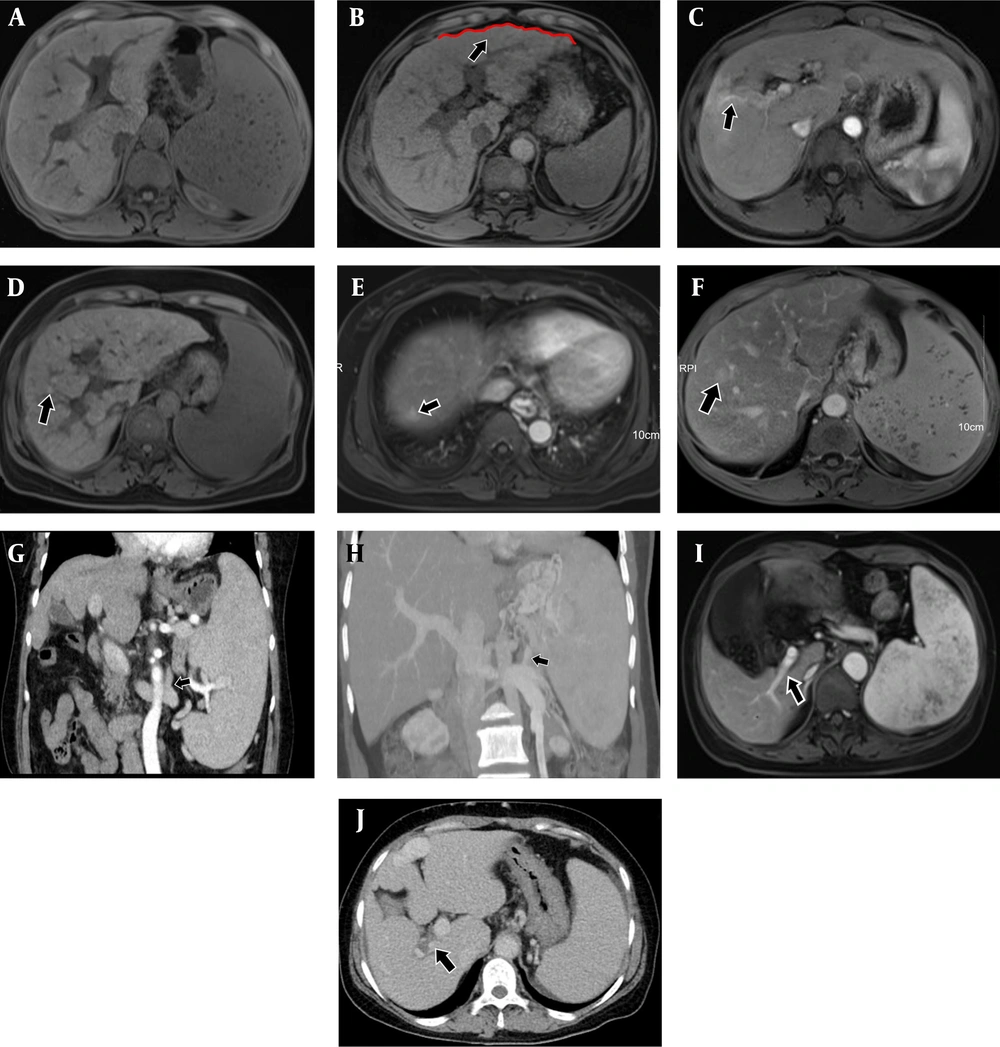
The liver of all fourteen patients (100%) in the IPH group and 21 patients (70%) in the cirrhosis group exhibited morphological changes. The majority of patients with IPH had smooth liver surfaces and thickened caudate lobes with "fist-like" changes (Figure 3A), while patients with cirrhosis mostly showed uneven surfaces with jagged changes (Figure 3B). Abnormal perfusion of the liver parenchyma around the portal vein was observed in 5 patients (35.7%) with IPH (Figure 3C), compared to only 3 patients (10%) in the cirrhosis group. Diffuse regenerative nodular manifestations on T1WI of the liver were seen in 8 patients (26.7%) in the cirrhosis group (Figure 3D), while none of the patients in the IPH group showed such manifestations. In 2 patients with IPH, FNH-like lesions were observed in the venous phase, which are benign lesions that enhance more in the venous phase than in the adjacent liver parenchyma on imaging (Figure 3E and F). However, FNH-like lesions were not present in the cirrhosis group (P > 0.05) (Table 2).
A, Magnetic resonance imaging (MRI)-T1WI.Idiopathic portal hypertension (IPH) patient with giant spleen, caudate lobe hypertrophy, smooth and slightly atrophic peripheral segments, and "fist-like" liver morphology in cross-section; B, Contrast enhanced MRI - arterial phase. Cirrhotic patient with uneven and "serrated" liver surface (black arrow); C, Contrast enhanced MRI-arterial phase. IPH patient with focal perfusion abnormalities in the arterial phase of the periportal liver parenchyma (black arrow). D, MRI-T1WI.cirrhotic patient with diffuse intrahepatic regenerative nodules on T1WI sequence (black arrow); E, F, Contrast enhanced MRI-venous phase. In 2 patients with IPH, FNH-like lesions could be observed in the venous phase (black arrow), a type of benign lesion that was more strongly enhanced in the venous phase than in the adjacent liver parenchyma on imaging; G, Contrast enhanced computed tomography (CT)-delay period. A patient with IPH whose tortuous gastric vein branches into the renal vein figure (black arrow); H, Portal CT angiography. A patient with IPH whose tortuous splenic vein branches converged into the renal vein (black arrow); I, Contrast enhanced MRI - arterial phase. In a patient with IPH, the right posterior branch of the portal vein was stiff and straight, and the distal branch of the portal vein was slender (black arrow); J, Contrast enhanced CT-delay period. A patient with IPH in the portal phase with a localized filling defect in the right secondary branch of the portal vein (black arrow)
4.2.3. Splenic-renal Shunts and Gastrorenal Shunts
Three patients with IPH had tortuous gastric veins branching into the renal vein (Figure 3G), and one patient with IPH had tortuous splenic veins branching into the renal vein (Figure 3H). In the cirrhosis group, two patients had tortuous gastric veins branching into the renal vein, and four patients had tortuous splenic veins branching into the renal vein (Table 2).
4.2.4. Abnormalities of the Portal Venous System
All 14 patients with IPH exhibited abnormalities in the portal venous system, characterized by widening of the main portal vein, stiffening and straightening of the portal vein (Figure 3I), and narrowing and occlusion of distal branch veins. In contrast, only 9 patients (30%) in the cirrhosis group showed abnormalities in the portal venous system, which manifested as thinning of the portal vein (P < 0.001). One patient in the IPH group experienced portal vein thrombosis (Figure 3J), while one patient in the cirrhosis group also experienced portal vein thrombosis (P = 0.0540) (Table 2).
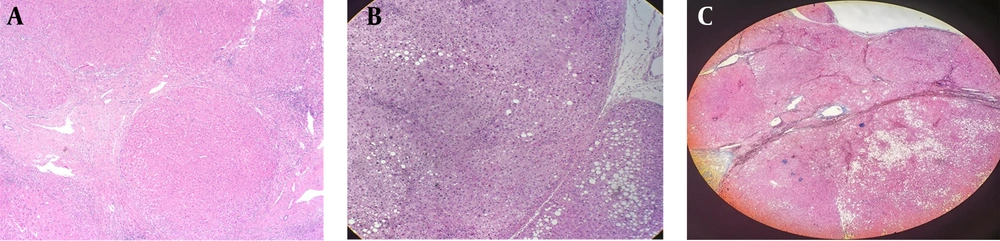
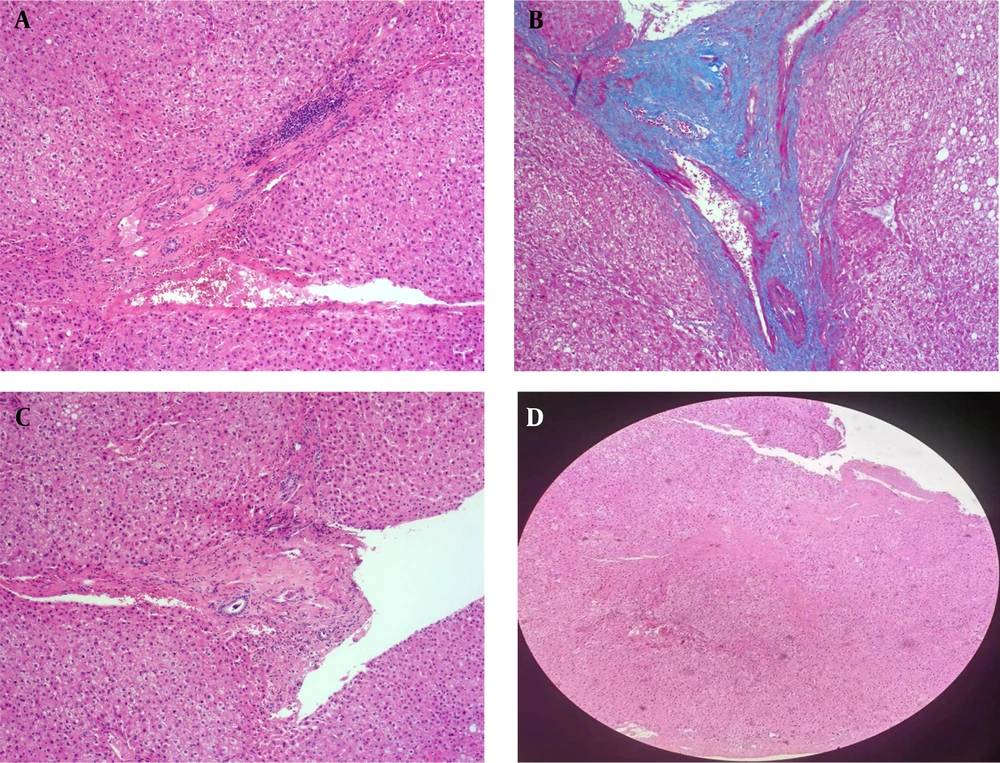
4.3. Pathological Changes in Patients with Idiopathic Portal Hypertension and Cirrhosis
The detailed pathological data of patients with IPH and cirrhosis are shown in Table 5. All patients with cirrhosis showed pseudolobule formation in histopathology, while this finding was not seen in IPH patients (P < 0.001). In addition, cell necrosis and edema were more frequent in cirrhotic patients compared to those with IPH (P < 0.001) (Figure 4A, B and C). Pathologically, patients in the IPH group displayed varying degrees of fibrosis in the portal areas (P < 0.001), with no significant lobular inflammation (P < 0.001) (Figure 5A and B). Some patients in the IPH group had portal vein occlusion and stenosis (Figure 5C and D), whereas patients in the cirrhosis group did not show obvious portal vein stenosis, occlusion changes, or fibrosis in the portal areas (P = 0.012).
| Parameter | Liver cirrhosis (n = 30) | IPH (n = 14) | X2 | P-Value |
|---|---|---|---|---|
| Pseudolobule formation | 30 (100) | 0 (0) | 39.511 | < 0.001 |
| Fibrosis in the portal areas | 0 (0) | 14 (100) | 39.511 | < 0.001 |
| Septal fibrosis | 30 (100) | 7 (50) | 14.296 | < 0.001 |
| Portal vein occlusion | 0 (0) | 4 (28.6) | 6.288 | 0.012 |
| Lobular inflammation | 26 (86.7) | 3 (21.4) | 17.029 | < 0.001 |
| Cell necrosis | 22 (73.3) | 2 (14.3) | 13.423 | < 0.001 |
Comparison of Pathological Features Between Patients with Idiopathic Portal Hypertension and Patients with Cirrhosis a
5. Discussion
Idiopathic portal hypertension is a rare clinical disease, and the epidemiological data on this condition vary by region, with more cases reported in Japan and India than elsewhere in Asia. The geographical and sex differences in the onset of IPH are still difficult to explain (2). A recently reported retrospective study of 115 patients with IPH in China found a male-to-female ratio of approximately 1: 1.25, with a high prevalence in the 40 - 60 year age group. There was also a significant time span between the first onset of symptoms and the final diagnosis of the disease, ranging from less than 1 year to as long as 20 years (6). The 14 patients included in this study had a male-to-female ratio of approximately 1: 1.8, which differs from the aforementioned reports, probably due to the low incidence of the disease. The average age of the patients was approximately 54 ± 8.9 years, and some patients had a longer time from the first clinical symptoms to diagnosis, consistent with the aforementioned reports. This shows that IPH in China is more prevalent in middle-aged individuals, more frequent in women, and more difficult to diagnose, which may be due to its atypical clinical presentation and lack of knowledge or experience regarding the disease.
Clinically, IPH and cirrhotic portal hypertension are similar in many ways. In clinical practice, IPH is often misdiagnosed as cryptogenic cirrhosis, but IPH is usually characterized by normal liver function, mildly elevated transaminases, and abnormal laboratory indicators such as a giant spleen, thrombocytopenia, and low white blood cells (2). In our study, patients in the IPH group also tended to have normal liver function, whereas functional indices were more likely to be abnormal in the cirrhosis group. This phenomenon may be because IPH is more prone to abnormalities of the portal venous system, whereas hepatocellular injury is more likely to occur in the cirrhotic group. Additionally, there were fewer leukocytes in the blood of IPH patients than in cirrhotic patients, which may be due to the more severe hypersplenism in IPH patients. In our study, TT and FG in the IPH group were significantly different from those in the cirrhosis group, and the blood of IPH patients was in a hypercoagulable state compared to that of the cirrhosis group. A clear diagnosis and selection of the correct treatment can improve the prognosis, so liver aspiration biopsy is very important for the definitive diagnosis of IPH. However, some unfavorable factors, such as hypersplenism and thrombocytopenia, may increase the risk of bleeding during liver aspiration biopsy, so it is important to find effective diagnostic methods to differentiate IPH from cirrhosis on imaging.
Since IPH is a rare disease, there is no standardized nomenclature for its pathological features thus far. Guido et al. (7) recommended the use of PV stenosis, herniated PV, hypervascularized portal tract, and periportal abnormal vessels to describe IPH.
Both IPH and cirrhotic portal hypertension imaging may show splenomegaly, varices, and changes in liver morphology. In the advanced stages of IPH, changes in contour and volume can occur. Similar to cirrhosis, IPH manifests as varying degrees of “wavy” changes or increased liver fissures (8, 9). However, the present study found that the liver capsule of the IPH group was smooth despite changes in the liver surface morphology. In contrast, the liver capsule in the cirrhosis group showed “wavy” changes and “jagged” roughness more often, demonstrating a difference in liver capsule morphology between the two groups. This may be caused by extensive fibrosis of cirrhotic liver tissue, necrosis or malnutrition of hepatocytes, atrophy of the liver envelope due to fibrous traction, and the generation of diffuse regenerative nodules on the liver surface. In contrast, patients with IPH may exhibit atrophy due to malnutrition of the peripheral liver parenchyma caused by atrophy and truncation of the fine peripheral branching portal veins. Additionally, the patients in the IPH group had an earlier disease course, which could contribute to the differences in liver capsule morphology between the two groups.
In terms of hepatic lobe atrophy and hyperplasia, the values of c/a (caudal right ratio) and c (coronal diameter of the caudate lobe) in the IPH group in this study were smaller than those in the cirrhotic group (P < 0.05), likely due to the narrowing and occlusion of the distal portal vein in IPH patients, resulting in reduced blood supply to the distal hepatic lobe and subsequent hepatocyte atrophy. Meanwhile, the caudate lobe, because of its unique blood supply (having independent arteries and short gastric veins), could develop compensatory hypertrophy or maintain normal morphology. In addition, the right lobe of the liver is less susceptible to toxin and viral damage in the IPH group than in the cirrhotic group, resulting in a reduced incidence of necrosis, fibrosis, and pseudolobular formation. Therefore, compensatory changes in the caudate lobe may be milder in the IPH group than in the cirrhotic group. The b value (degree of hyperplasia of the left outer lobe of the liver) was also lower in the IPH group than in the cirrhotic group, probably because IV segment atrophy and caudate lobe hypertrophy are often observed in cirrhotic patients (10). In the early stages of the disease, when the liver is not decompensated, the left outer lobe of the liver may enlarge compensatorily. In patients with IPH, the left outer lobe of the liver atrophies due to insufficient blood supply from the fine branching portal veins.
In terms of splenomegaly, an early study of portal venous dynamics in IPH classified patients into two groups: One with significantly increased splenic and portal venous flow and the other with significantly increased portal vascular resistance and portal venous pressure (11). A dual hypothesis has been proposed based on these findings, namely, increased splenic blood flow and occlusion of small- to medium-sized portal branches. In the first case, diffuse high expression of inducible nitric oxide (NO) synthase and endothelial NO synthase is observed in the sinusoidal endothelial cells of the spleen; there is splenic sinus enlargement and splenomegaly, leading to increased splenic venous blood flow and elevated portal venous pressure. In the second case, the occluded portal vein is the characteristic pathological manifestation of IPH, suggesting that diseases causing damage to small and medium-sized portal branches may be the underlying cause of IPH (11). However, the most common causes of liver cirrhosis in China are HBV, HCV, and alcohol (12). The main pathological changes in IPH involve fiber hyperplasia and hepatocyte proliferation in the hepatic lobules. The hyperplastic fibers and regenerative nodules narrow and obstruct the hepatic sinusoids, causing portal hypertension by impeding portal blood flow into the central vein of the hepatic lobules. This leads to portal blood flow stagnation in the spleen and results in secondary splenomegaly. In terms of the pathogenesis of IPH and cirrhosis, splenomegaly due to cirrhosis is a long-term process, whereas giant spleen-like manifestations can occur early in the pathogenesis of IPH. In this study, splenic thickness and length were significantly greater in IPH patients than in cirrhotic patients (P < 0.05), but confirmation in a larger cohort is needed, along with clarification of the relevant clinical staging of both diseases.
Both cirrhosis and IPH can lead to portal hypertension, resulting in the development of varices and subsequent formation of spontaneous portosystemic shunts (SPSSs), including gastro-renal shunts (GRSs) and splenorenal shunts (SRSs). In this study, there were 4 patients with SPSS in the IPH group and 6 patients with SPSS in the cirrhosis group, with GRS:SRS ratios of 3:1 and 2:4, respectively. However, these differences did not reach statistical significance. Currently, there is limited literature on spontaneous shunts related to IPH, making it challenging to differentiate between the two diseases based on the type of shunt.
Among the parenchymal-related changes, diffuse intrahepatic regenerative nodules occurred in four patients with cirrhosis, while this sign was not found in patients with IPH, consistent with the report by Zhao et al. (13). This finding pathologically corresponds to diffuse fibrosis, diffuse necrosis, and nodular regeneration of hepatocytes in cirrhosis. In contrast, cellular necrosis rarely occurs in IPH pathology despite the presence of fibrosis in the portal area. Intrahepatic focal nodular hyperplasia-like lesions were found in 2 patients with IPH, while this sign was not found in the cirrhotic group, consistent with previous reports (10, 14). Focal nodular hyperplasia-like lesions occur in IPH due to reduced portal blood supply and increased compensatory arterial blood supply. Because of increased perfusion, patchy abnormal enhancing shadows around the portal vein were found in five patients with IPH in the arterial and venous phases, while abnormal perfusion was found in only one patient in the cirrhotic group. This is likely because the portal vein is less affected in cirrhotic patients than in IPH patients.
Six patients in the cirrhosis group in our study had hepatocellular carcinoma (HCC), but only one patient had invasion of the portal vein. HCC can affect portal blood supply through cancerous thrombi or direct invasion. The presence of focal nodular hyperplasia-like lesions and the absence of diffuse regenerative nodules should raise suspicion of IPH (10).
All 14 patients with IPH exhibited abnormalities in the portal venous system, characterized by widening of the main portal vein, stiffening and straightening of the portal vein, and narrowing and occlusion of distal branch veins. In the cirrhosis group, 9 patients showed abnormalities in the portal venous system, manifested as thinning of the portal vein. Eight of these cases were due to hepatitis B cirrhosis, and one case was familial hereditary cholestasis syndrome. The main cause of abnormalities in the portal system of IPH is fibrosis in the portal area, which leads to widening of the main trunk of the IPH portal vein and narrowing or even loss of branches, resulting in a series of changes in the liver. In hepatitis B and cholestasis syndrome, portal vein slenderness is caused by long-term chronic inflammatory infiltration and hepatocyte necrosis, enlargement, and degeneration, especially compression of normal portal vessels in the liver during pseudolobule formation.
Regarding portal vein thrombosis, there was one patient each in the IPH and cirrhosis groups. According to other scholars' reports, the probabilities of portal vein and major branch abnormalities and extrahepatic thrombosis in IPH were 58% and 43%, respectively (15), whereas 93% of patients with cirrhosis did not have occlusion of the portal system both inside and outside the liver (16). The discrepancy in this study may be due to the small sample size of the IPH group. The greater probability of portal thrombosis in IPH compared to cirrhosis may be because portal hypertension is much greater in IPH patients than in cirrhosis patients (16), and higher portal pressure is more likely to cause endothelial damage leading to thrombosis.
In the cirrhosis group, patients exhibited cell necrosis, edema, and pseudolobule formation, while patients in the IPH group did not exhibit pseudolobule formation. Pathologically, patients in the IPH group displayed varying degrees of fibrosis in the portal areas, with no significant lobular inflammation. Some patients in the IPH group had portal vein occlusion and stenosis, whereas patients in the cirrhosis group did not show obvious portal vein stenosis or occlusion changes. Pseudolobule formation is a characteristic change in cirrhosis. Under the influence of chronic inflammation and venous return obstruction, diffuse necrosis of hepatic parenchymal cells occurs, leading to nodular proliferation of hepatocytes and regenerative nodule formation. These regenerative nodules are surrounded by fibers to form pseudolobules. The essence of IPH is still presinusoidal portal hypertension caused by portal vein fibrosis, with the main lesion located in the portal vein. The surrounding liver lobular and parenchymal lesions in IPH occur as secondary changes.
This study has several limitations. Retrospective studies include a risk of selection bias. The lack of validation controls can affect the validation results. The number of cases was small, and more measurements would have helped reduce errors. The amount of liver lobe atrophy and hyperplasia may have been inaccurate due to the simple measurement of cross-sectional imaging.
Although portal hypertension in IPH is severe, liver function tends to appear normal compared to cirrhosis, and hypersplenism may lead to a decrease in platelets, red blood cells, and white blood cells. In patients with early IPH, if a giant spleen is found on imaging and the liver surface is smooth, IPH should be considered. While focusing on changes in the portal system through imaging, the caudal right ratio of the liver and hyperplasia and atrophy of the left outer lobe of the liver must be given some reference importance. This requires measuring the coronal diameter of the liver on transverse axial images. The values of the caudal right ratio, coronal diameter of the caudate lobe, and coronal diameter of the left lobe of the liver in IPH are regularly smaller than those in cirrhosis. In addition, focal nodular hyperplasia-like lesions and diffuse hepatic regenerative nodules can also be used as key points to differentiate IPH from cirrhosis. The pathological absence of pseudolobular changes and the presence of abnormal changes such as fibrosis, stenosis, and occlusion of the portal venous system require cautious consideration for diagnosing cirrhosis.
At present, liver biopsy remains the "gold standard" for diagnosing IPH. As research on IPH progresses worldwide, a more accurate diagnosis of IPH can be made in the future by combining imaging with biochemical and other noninvasive tests to establish a histological model.