1. Background
Hypertension is defined as systolic tension or diastolic tension values ≥ 140 mmHg and ≥ 90 mmHg, respectively. It is a significant risk factor for stroke, coronary heart disease, heart failure, and renal and vascular diseases (1). Renal artery stenosis (RAS) almost inevitably leads to high blood pressure, resulting in renovascular hypertension (RVH). The decrease in renal blood flow causes ischemia in the kidneys, which in addition to the RVH can result in ischemic nephropathy or end-stage renal failure. The early diagnosis of RAS allows correction of secondary hypertension (RVH) in the majority of patients. RAS is most commonly due to atherosclerosis, the prevalence of which increases with age. In young females, however, fibromuscular dysplasia of the arterial wall is the most characteristic finding (2). Physiological tests and radionuclide imaging do not provide high levels of diagnostic efficiency in the diagnosis of RAS. While renal color Doppler ultrasound (CDU) is an efficient diagnostic method in patients with RAS, it may not be applicable in obese patients and patients with abdominal distension due to intestinal gas. Computerized tomography (CT) angiography, magnetic resonance angiography (MRA), and digital substractional angiography (DSA) are more effective in assessing renal arteries. However, all of them require the use of contrast materials that may be nephrotoxic. In addition, CT angiography and DSA involve the use of ionizing radiation (2-7).
In recent years, non-contrast MRA (NC-MRA) has become an important diagnostic method that does not involve the use of contrast media or ionizing radiation. An additional advantage is its high resolution (8-11). A limited number of studies involving small numbers of patients have demonstrated that NC-MRA offers an alternative to contrast-enhanced magnetic resonance angiography (CE-MRA).
2. Objectives
The aim of the present study was to determine the efficiency of NC-MRA when used with the inflow inversion recovery (inhance) method in patients with hypertension undergoing assessment of the renal arteries.
3. Patients and Methods
3.1. Patients
The study population consisted of 66 adult patients admitted to our hospital’s radiology department between Feburary 2015 and March 2016 with findings of hypertension and normal kidney function and a suspicion of RAS.
Patient selection was based on the American Cardiology College/American heart association’s 2005 practice guidelines RVH risk index: The following patients were included: (i) those with an onset of severe or stage II hypertension after the age of 55 (blood pressure > 160/100), (ii) those with refractory or persistent hypertension, (iii) those in whom the use of three appropriate therapeutic doses of antihypertensive drugs (with diuretics) failed to maintain adequate blood pressure control, (iv) those with a sudden onset of severe hypertension; and (iv) those with well-managed hypertension who experienced a sudden increase in blood pressure. Other included patients were: (i) hypertensive patients with abdominal murmur; (ii) smokers who had mild to severe hypertension, pervasive atherosclerosis, or consistently high levels of creatine; (iii) hypertensive patients with onset before 30 years of age; and (iv) hypertensive patients with atherosclerosis and progressive kidney failure who were resistant to complicated therapy. Patients considered to be at risk of RVH based on the RVH index adopted for this study (12) were those with: (i) malignant hypertension (end-organ damage accompanying severe hypertension: acute kidney failure, retinal hemorrhage, papilledema, heart failure or neurologic disorders) and severe hypertension (diastolic blood pressure > 120 mmHg); (ii) a sudden elevation of plasma creatine concentration shortly after starting single angiotensin-converting enzyme inhibitor therapy or angiotensin 2 receptor blockers; (iii) hypertension accompanied by the new onset of unexplained elevated levels of creatine; and (iv) mild-severe hypertension and an unexplained atrophic kidney asymmetry or an asymmetry with respect to kidney size (> 1.5 cm).
The patients’ renal arteries were assessed by color Doppler ultrasound (CDU) and then on the same or following day with both NC-MRA using the inhance technique and three-dimensional (3D) CE-MRA. The images were read by two radiologists (with 5 and 6 years of experience in MRA) and the resulting data were assessed statistically. Among the 66 patients with suspected RVH, 28 were female and 38 were male. They ranged in age from 18 to 85 (median age 51.2) years. The patients’ demographic data and their CDU, 3D CE-MRA, and NC-MRA findings were reviewed.
Patients with renal artery stenosis detected on NC-MRA and/or CE-MRA underwent DSA for the detection and treatment of RSA.
3.2. Ethical Considerations
All of the patients were informed about the nature and intent of the investigation and required to provide written informed consent to participate in the study. The study was approved by the ethics committee of our hospital (project number: KA 15/295).
3.3. MR Protocol
Patients with a normal kidney function test underwent a MR examination following 6 hours of fasting. During the same session, they also underwent NC-MRA and CE-MRA examinations. All MR examinations were performed using a 1.5-T whole-body imaging MR system (Optima 360; GE Healthcare, Milwaukee, WI, USA) with a standard 16-channel phased-array body coil. 3D CE-MRA was conducted using 3D spoiled gradient-echo sequences and gadolinium-based contrast material (0.2 mmol/kg Optimark, Mealis; 2 mL/s injected automatically + 20 mL saline). NC-MRA was conducted using respiratory-triggered 3D fat-saturated fast imaging with steady state acquisition (FIESTA) and inversion recovery pulses (inhance 3D inflow inversion recovery, IR). Table 1 shows the NC-MRA and CE-MRA parameters. Dual-echo sequences were added to the MR examination for the assessment of the adrenal glands in four patients.
| Variables | NC-MRA (Inhance) | CE-MRA |
|---|---|---|
| TE, ms | 2.2 | 1.3 |
| TR, ms | 4.4 | 3.6 |
| Flip angle | 60 | not applicable |
| TI, ms | 175 | |
| Blood sup TI, ms | 1200 | not applicable |
| Receiver bandwidth, Hz/pixel | 125 | 83.33 |
| Field of view | 34.0 | 42.0 |
| Slice thickness, mm | 1.6 | 2.8 |
| Frequency matrix | 224 | 384 |
| Phase matrix | 320 | 256 |
| Phase field of view | 1.00 | 0.95 |
| Acquisition time | 4.40 - 6.55 min (1 or 2 respiratory intervals) | 16 - 22 s |
| NEX | 1 | 1 |
| Locations per slab | 100 | 40 |
| Voxel size, mm3 | 1.5 × 1.06 × 1.6 | 0.09 × 1.64 × 2.8 |
| Contrast dose | not applicable | 0.2 mmol/kg (Optimark, Mealis) 2 mL/s automatic injection + 20 mL saline |
Sequence Parameters of Non-Contrast Magnetic Resonance Angiography (Inhance) and Contrast-Enhanced Magnetic Resonance Angiography
3.4. MRA Data Analysis
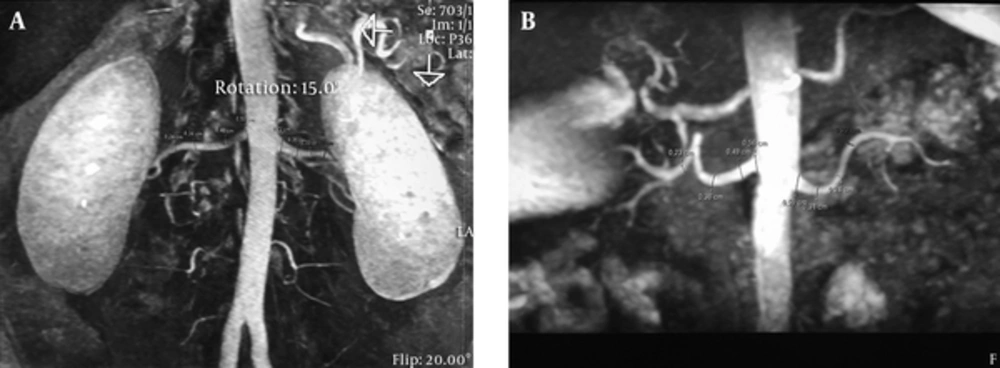
Two readers blinded to the 3D CE-MRA findings or other clinical information of the patients evaluated the NC-MRAs independently on a PACS (picture archiving communication systems, clearcanvas) workstation, including access to reformatted views and 3D maximum intensity projection and volume rendered images. The images were magnified four-fold and segments at the level of origin of the renal arteries and beyond were separated into three equal parts. Reformat measurements were taken at the exact mid-point (Figure 1).
3.5. Image Assessment
3D CE-MRA served as the gold standard for diagnosing RAS, which was graded as mild (1% - 49%), moderate (50% - 69%), severe (70% - 99%), or occlusion (100%) (3). The assessment of the right renal artery in one patient with a renal artery stent was disregarded.
The quality of the NC -MRA and 3D CE-MRA images was graded as follows:
Excellent: Homogeneous signal intensity in the vessel without flow artifacts; sharp and complete delineation of the vessel borders; minimal interference from the venous system.
Good: Homogeneous signal intensity in the vessel, with slight flow artifacts; good delineation of the vessel borders; coverage of the main renal artery and segmental branches up to the renal parenchyma.
Poor: Inhomogeneous signal intensity in the vessel; irregular delineation of the vessel borders; unclear depiction of the main renal artery.
Not assessable: Vessels not visible or diagnostic information could not be obtained due to severe blurring artifacts.
3.6. Statistical Methods
The data were assessed using SPPS 20 (released 2011. IBM SPSS Statistics for Windows, version 20.0. IBM Corp., Armonk, NY). The median ± standard deviation and the median (range) ratio and frequency were determined after confirmation of the normality and homogeneity of the data (Shapiro-Wilk and Levene tests). A paired t test (Student’s t test) was used to compare independent groups; otherwise, a Mann-Whitney U test was used. A P value < 0.05 or < 0.01 was considered to indicate statistical significance. Within group intraclass correlation coefficients (ICC) were also determined. Additional statistical methods consisted of Fisher’s exact and χ2 tests.
4. Results
Among the 66 patients undergoing NC-MRA and 3D CE-MRA examinations, 126 main renal arteries and 12 accessory arteries were assessed. Reader-1 and reader-2 did not significantly differ in their assessments of the images produced by the two imaging modalities with respect to diameter measurements of the ostium and segments of the renal arteries (Table 2). Inter-reader agreement regarding the NC-MRA and 3 D CE-MRA findings was either good or excellent (ICC = 0.75 - 0.94) for all segments, with a coefficient confidence interval of 0.57 - 0.96 (Table 3). In the qualitative analysis of both NC-MRA and 3D CE-MRA, reader 1 rated 89.3% and reader 2 rated 87.5% of the images as good or excellent. Table 4 shows the image quality findings reported by the two readers.
| Variables | NC-MRA (Inhance)a | CE-MRAa | P Value |
|---|---|---|---|
| Right Renal Artery | |||
| Origin | |||
| Reader-1 | 6.04 ± 1.60 | 5.87 ± 1.40 | 0.87 |
| Reader-2 | 5.82 ± 1.32 | 5.68 ± 1.40 | 0.2 |
| Proximal 1/3 | |||
| Reader-1 | 4.41 ± 1.18 | 4.28 ± 1.08 | 0.09 |
| Reader-2 | 4.56 ± 1.17 | 4.43 ± 1.03 | 0.12 |
| Middle 1/3 | |||
| Reader-1 | 3.85 ± 1.08 | 3.69 ± 1.01 | 0.4 |
| Reader-2 | 4.22 ± 0.96 | 4.11 ± 0.88 | 0.06 |
| Distal 1/3 | |||
| Reader-1 | 3.31 ± 1.03 | 3.21 ± 0.95 | 0.4 |
| Reader-2 | 3.67 ± 0.85 | 3.50 ± 0.82 | 0.07 |
| Left Renal Artery | |||
| Origin | |||
| Reader-1 | 6.39 ± 1.34 | 6.17 ± 1.26 | 0.07 |
| Reader-2 | 6.37 ± 1.47 | 6.19 ± 1.28 | 0.1 |
| Proximal 1/3 | |||
| Reader-1 | 4.39 ± 1.34 | 4.48 ± 0.93 | 0.10 |
| Reader-2 | 4.81 ± 1.24 | 4.63 ± 1.09 | 0.06 |
| Middle 1/3 | |||
| Reader-1 | 4.10 ± 1.04 | 3.89 ± 0.77 | 0.06 |
| Reader-2 | 4.43 ± 1.20 | 4.24 ± 1.08 | 0.07 |
| Distal 1/3 | |||
| Reader-1 | 3.60 ± 1.03 | 3.38 ± 0.81 | 0.06 |
| Reader-2 | 3.94 ± 1.09 | 3.74 ± 0.93 | 0.07 |
Renal Artery Diameter Data of Patients (Readers 1 and 2)
| Parameter | ICC (average measurement) | %95 CI of ICC | ||
|---|---|---|---|---|
| NC-MRA | CE-MRA | NC-MRA | CE-MRA | |
| Right renal artery | ||||
| Origin | 0.87 | 0.94 | 0.77 - 0.92 | 0.90 - 0.96 |
| Proximal | 0.85 | 0.90 | 0.75 - 0.91 | 0.83 - 0.94 |
| Middle | 0.80 | 0.85 | 0.68 - 0.88 | 0.75 - 0.91 |
| Distal | 0.76 | 0.88 | 0.60 - 0.86 | 0.80 - 0.93 |
| Left renal artery | ||||
| Origin | 0.93 | 0.81 | 0.87 - 0.95 | 0.67 - 0.88 |
| Proximal | 0.81 | 0.81 | 0.68 - 0.89 | 0.68 - 0.89 |
| Middle | 0.77 | 0.82 | 0.61 - 0.87 | 0.70 - 0.89 |
| Distal | 0.75 | 0.75 | 0.57 - 0.86 | 0.57 - 0.86 |
Inter-Rater Agreement and Readability Analysis
| Image Quality | Reader 1 | Reader 2 | ||
|---|---|---|---|---|
| NC-MRA | CE-MRA | NC-MRA | CE-MRA | |
| Right side (n = 63) | ||||
| 1- (not assessable) | 5 (7.9) | 2 (3) | 5 (7.9) | 2 (3) |
| 2- (poor) | 2 (3) | 1 (1.5) | 3 (4.7) | 2 (3) |
| 3- (good) | 25 (39) | 6 (9.5) | 24 (38) | 5 (7.9) |
| 4- (excellent) | 31 (49.2) | 54 (85.7) | 31 (49.2) | 54 (85.7) |
| Left side (n = 63) | ||||
| 1- (not assessable) | 4/66 (6.3)b | 2 (3) | 5 (7.9) | 2 (3) |
| 2- (poor) | 3 (4.7) | 2 (3) | 3 (4.7) | 2 (3) |
| 3- (good) | 21 (33.3) | 2 (3) | 24 (38) | 5 (7.9) |
| 4- (excellent) | 35 (63) | 57 (90.4) | 31 (49.2) | 54 (85.7) |
Qualitative Analysis of Image Quality in Non-Contrast-Enhanced and Contrast-Enhanced Magnetic Resonance Angiographya
Twelve accessory renal arteries were observed with CE-MRA in seven patients. In the assessment of accessory renal arteries, 3D CE-MRA was superior to NC-MRA, as in the latter only seven out of 12 accessory renal arteries (58%) were detected.
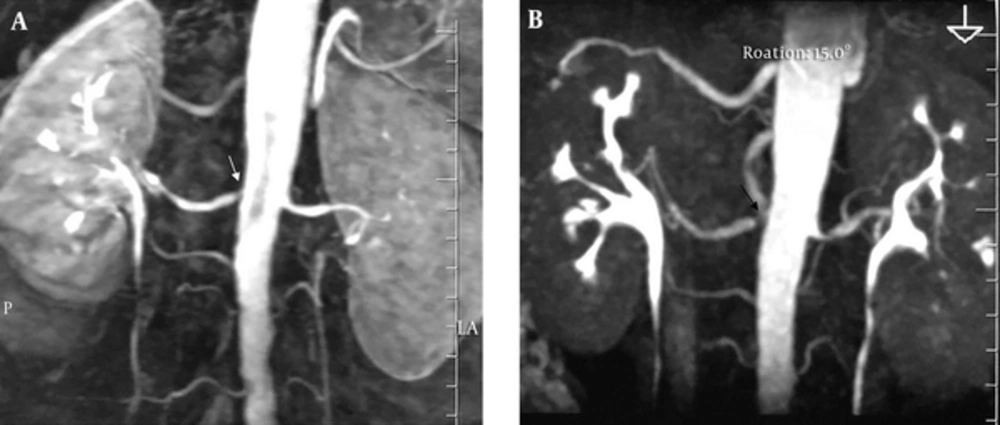
3D CE-MRA or catheter angiography was performed to detect stenosis in five patients. In renal arteries in which a stenosis was identified using DSA and CE-MRA, the finding was confirmed by NC-MRA. However, compared with the other imaging techniques NC-MRA slightly overstimated the degree of stenosis (Figure 2).
Several types of artifacts were identified that had an effect on resolution, the most common of which was motion artifacts resulting from poor breath-holding or imperfect respiratory gating. Less common artifacts were parallel imaging reconstruction (NC-MRA), susceptibility artifact from adjacent gas or metal (NC-MRA and 3D CE -MRA), reduced arterial signal (NC-MRA), and venous contamination (NC-MRA and 3D CE-MRA).
Adrenal adenoma was diagnosed in five patients. In one patient, the adenoma was bilateral. A kidney mass was observed incidentally in three patients, two of whom were operated on following a histopathologic diagnosis of renal cell carcinoma.
5. Discussion
The early diagnosis of RAS is critical to protecting renal function and in the management of hypertension. The inability of CDU to image all segments of renal and accessory renal arteries has encouraged the development of new techniques. Of these, renal MRA has the advantage of a strong diagnostic quality and the absence of radiation exposure (4-6). 3D CE-MRA can be used to evaluate the vascular structures in nearly all of the body. T1-weighted spoiled gradient-echo sequences and central k-space acquisition during the arterial phase of the study maximize the preferential visualization of the arteries, while the use of Gd-based contrast can shorten the T1-interval of blood that otherwise seems bright (13). However, the risk of nephrogenic systemic fibrosis due to a gadolinium reaction or kidney dysfunction in patients with hypertension hinders the use of contrast-based examinations (11, 14, 15). Moreover, recent studies have suggested that gadolinium can accumulate in the brain (16, 17). Hence, alternative MRA techniques allowing imaging of the renal arteries without the need for contrast material have been developed (9-11, 18, 19), such as true fast imaging with steady-state precession (TrueFISP) MRA, repetitive artery and venous labeling (RAVEL), and inhance.
The safety of NC-MRA techniques has been evaluated in comparative studies with CE-MRA. In a study comparing the TrueFISP technique and CE-MRA, there was no significant difference in the determination of main renal artery volume, maximal visible renal artery length, and number of branches. However, a stenosis score of 10 in RAS patients was more frequent using CE-MRA than TrueFISP MRA. In the qualitative scoring, TrueFISP MRA was significantly better than CE-MRA (P < 0.05) (19).
Park et al. (9) used NC-MRA at 3 T with the RAVEL technique to visualize the renal arteries, an approach that yielded an acceptable overall image quality (fair or better image quality in 88% of right and 96% of left renal artery images). The diagnostic performance was excellent (100%) with respect to determining the number of renal arteries. The sensitivity and specificity in detecting the presence or absence of early branching vessels varied from 82% to 100%.
In our study, inter-reader agreement regarding the NC-MRA findings was moderate or good for all segments except the right distal artery, in which the findings were similar to those of CE-MRA.
Better image quality was achieved for the left compared to the right side, which may have been due to the less suppressed background signal of the right renal arteries on NC-MRA combined with the RAVEL technique. Although contrast material is not used in the inhance technique, the diagnostic quality in evaluations of the arterial structures was high.
Inhance is an angiographic sequence technique that was developed to provide consistent, reproducible images of the renal arteries while completely repressing signals from static background tissue and venous blood. Inhance inflow IR combines the advantages of the inflow influences of time-of-flight (TOF) MRA with those of the bright luminal signal of fast imaging employing steady state acquisition (FIESTA) sequences. The two are integrated with an IR pulse to repress the venous and background tissue signals. The 3D FIESTA-based application yields high-quality 3D bright blood images with a considerably increased signal-to-noise ratio. A selective inversion pulse is conducted over the region of interest that inverts the magnetization of the arterial and venous blood as well as that of static tissue. During magnetization recovery, another pulse is conducted at the time of the null point of venous blood, to sample the arterial signal. The net result is an angiographic image with sound background suppression and without venous contamination. Spectrally selective IR fat suppression using an adiabatic radiofrequency pulse is applied to yield uniform fat suppression, while respiratory gating minimizes respiratory motion artifacts, allowing free-breathing MRA of the renal artery (18).
In their study using the inhance technique, Glocker et al. (11) reported good agreement between NC-MRA and 3 D CE-MRA. They concluded that inhance offers an alternative imaging approach in patients with suspected RVH who are not eligible for CE-MRA. They also determined that NC-MRA in RAS patients overestimates the degree of stenosis compared to 3D CE-MRA. Among the possible explanations for this result were pulse sequence limitation depending on respiratory motion, parallel imaging reconstruction artifacts, lower spatial resolution, partial volume averaging in NC-MRA vs. secondary 3D CE-MRA, and differences in the acquisition planes (axial NC MRA vs. oblique coronal 3 D CE-MRI).
In our study, the degree of stenosis based on the NC-MRA images was slightly overestimated in some cases. In a recent animal study, Bley et al. (8) determined that this overestimation was acceptable and would not impact patient management. In line with the results of previous studies, we did not find significant differences between NC-MRA and 3D CE-MRA with respect to identifying stenosis, image quality, or diagnostic quality. Among our patients, there were none who could not be assessed due to the above-described limitations of the inhance technique.
The limitations of NC-MRA are the use of respiratory triggering, sensitivity encoding (SENSE)-based or sensitivity-encoding parallel imaging array spatial sensitivity encoding technique (ASSET), and restricted volumetric coverage. Phase ghosting artifacts arising from the respiratory trigger can be avoided using navigator gating; whereas focal artifacts due to the use of SENSE-based or ASSET can be managed by a phase field of view large enough to cover the entire diameter of the abdomen, although this will somewhat limit the achievable spatial resolution. Restricted volumetric coverage is another limitation. In patients with a large aortic inflow volume, this can be dealt with through an adjustable TI.
NC-MRA was reported to perform better than 3D CE-MRA in the imaging of intrarenal segmental arterial branches. Segmental renal artery imaging is of diagnostic value in patients with dysplasia or vasculitis involving peripheral renal arterial branches (11). Because stenosis in the accessory renal arteries can be a cause of hypertension, their identification and assessment are important (7). In our study, 3D CE-MRA was more efficient than NC-MRA in the assessment of the accessory renal arteries. Our results were compatible with those of a previous study using the inhance technique. In that study, on the CE-MRA images, one reader missed five accessory arteries and the second reader missed eight. The readers proposed that the free precession acquisitions in combination with respiratory-gated sequences and steady-state breath-holding that are characteristic of NC-MRA examinations may improve imaging of the inferior accessory renal arteries and thus diagnostic accuracy (11, 20).
Our inability to compare the DSA findings with those of the other imaging modalities in a significant number of patients was a major limitation of our study. However, in previous studies, 3D CE-MRA yielded results similar to those of DSA and its safety was confirmed. Another limitation of our study was the limited number of patients with RAS. Further studies based on a large patient series will be better able to evaluate the role of inhance in diagnosing stenosis.
In conclusion, our study identified a strong correlation between inhance and 3D CE-MRA sequences. Inhance sequences are of high performance in the imaging of the renal arteries and in obtaining homogenous images in of the venous lumen. Thus, in assessments of the renal arteries, NC-MRA using inhance sequences offers an alternative to MRA sequences. The failure to detect RAS using the inhance method may obviate the need for CE-MRA or DSA.