1. Background
Femoral artery pseudoaneurysm (FAP) is the most common complication after endovascular procedures, which occurs in 0.5 - 9% of patients, according to some series (1). Femoral artery pseudoaneurysm can cause persistent pain and swelling in the groin, compression neuropathy, limb ischemia, skin necrosis, and even sudden rupture (2-4). These complications are more likely to occur in individuals with a higher Body Mass Index (BMI) and those taking antiplatelet and anticoagulant medications, especially patients with cardiovascular disease (5). The growing tendency to use less invasive methods in recent decades has significantly increased the number of endovascular procedures that lead to FAP (6, 7).
So far, various interventions, including surgery, ultrasound-guided compression, and ultrasound-guided thrombin injection (UGTI), have been proposed for FAP treatment (3, 5). However, there are still significant challenges in treating FAP, including low success rate, procedural difficulty, and significant complications (2). Ultrasound-guided compression repair is more widely accepted among the proposed methods due to its noninvasive nature and relatively high success rate. However, the therapeutic outcomes of this treatment are negatively influenced by its time-consuming and laborious procedure and its technical problems for radiologists (8). Therefore, modifications in compression methods to assume better control over the stability and location of the pressure and the patient’s pain can improve the applicability of this noninvasive method (2, 5). Previously, few alternative techniques, including the use of C-arm clamps (9), the FemoStop compression device (10), and stethoscope-guided compression (11), have been proposed for the compression repair of pseudoaneurysms.
In the present study, we investigated the efficacy of a novel compression device in improving the ultrasound-guided compression repair of angiography-induced pseudoaneurysms.
The ratchet mechanism in the device allows continuous linear motion in only one direction while preventing motion in the opposite direction. It also makes it possible to apply controlled constant high pressure on the compression site while monitoring the blood flow in the pseudoaneurysm and the distal limb. The device is also mounted on the bedside, and the patient's movement does not interfere with the procedure. Due to the constant magnitude and localization of the pressure applied by the device, a higher success rate may be expected.
2. Objectives
The present study aimed to evaluate the efficacy of a new device in treating angiography-induced FAP compared to the conventional manual compression.
3. Patients and Method
3.1. Research Design
The research was designed as a randomized clinical trial. The study was blinded in terms of clinical evaluation and statistical analyses. The trial was performed on patients with angiography-induced FAP in the Radiology Department of Ghaem Hospital, Mashhad, Iran, from October 2017 to March 2019. This article was composed according to the Consolidated Standards of Reporting Trials guidelines.
3.2. Inclusion and Exclusion Criteria
The inclusion criterion was the diagnosis of a femoral angiography-induced pseudoaneurysm within 72 hours of angiography, which was verified using color Doppler ultrasound. The exclusion criteria were the presence of complete blockage, complete pseudoaneurysm thrombosis, abscess, surface tissue infection, open wound in the area, and the lack of consent to enrollment.
3.3. Sampling, Blinding, and Allocation Method
The sample was formed using purposive sampling. The sample size was estimated to be n = 11 per group (minimum) using the sample size formula for comparing two proportions (rate of success in FAP treatment) with the assumptions: Two-sided α = 5%, test power = 80%, P1 = 0.45, and P2 = 0.90. Patients were assigned to the intervention group (ultrasound-guided compression with the invented device) or the control group (manual ultrasound-guided compression) at random using a sealed envelope method. To ensure blinded assignments, random numbers were obtained from https://www.random.org, and a colleague outside the research team was asked to place one number in each envelope. An envelope was randomly drawn for each eligible patient, and the patient was assigned to one of the groups based on the envelope's contents. An outside radiologist performed the recovery assessment ultrasound to make the research double-blinded, and the results were handed to the researchers in coded form. The data was entered into the software in coded form, and the statistical analyst was not aware of the group details.
3.4. Data Collection
The data regarding age, gender, BMI, the time between angiography and the visit for pseudoaneurysm treatment, and the status of anti-thrombotic treatment, including medication with heparin, were recorded at the beginning of the study. The patients then underwent an ultrasound examination of the femoral area to determine the size of the pseudoaneurysm and the ratio of pseudoaneurysm thrombosis to open lumen. After the interventions, the duration of successful treatments, the pain scores, and the success rate of the interventions were recorded. The patients were followed up for 6 hours after the procedure to record the incidence of unforeseen complications.
3.4.1. Ultrasound
All ultrasound exams were performed by an expert radiologist with 15 years of experience using a Samsung Medison SonoAce X8 ultrasound system with curved (2 – 8 MHz) and linear (5 – 12 MHz) probes. The dimensions of the pseudoaneurysm were recorded in the format of length × width in millimeters. The size of the pseudoaneurysm neck was also recorded in millimeters. The ratio of pseudoaneurysm thrombosis to open lumen was recorded in percentage points before the procedure.
3.4.2. Therapeutic Success
Therapeutic success, defined in percentage points, was determined as follows. The patients underwent a second femoral ultrasound 20 minutes after the intervention to assess the blood flow in the pseudoaneurysm. In cases with complete thrombosis, the treatment was considered successful, and the procedure was completed. In cases without complete lumen thrombosis, the procedure was repeated up to two more times, each for 20 minutes. The treatment was considered a failure if complete lumen thrombosis was not observed after the third repeat. The duration of the intervention was considered as the time from the onset of the procedure until the termination of blood flow in the pseudoaneurysm while observing the blood flow in the femoral artery.
3.4.3. Pain Score
The pain score during compression was measured using the Visual Analog Scale (VAS) (12). To record the pain intensity, the patient was asked to rate the pain on a scale of 0 - 10 on a ruler, with 0 representing “no pain” and 10 representing “the most severe pain.” The patient’s report of pain severity during the procedure was recorded on a scale of 0 to 10.
3.4.4. Follow-up and Complications
The patients were monitored in the CCU for 6 hours after the intervention to record any side effects.
3.5. Interventions
3.5.1. Ultrasound-Guided Compression with the Invented Device
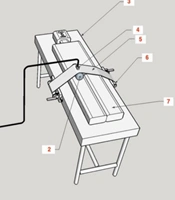
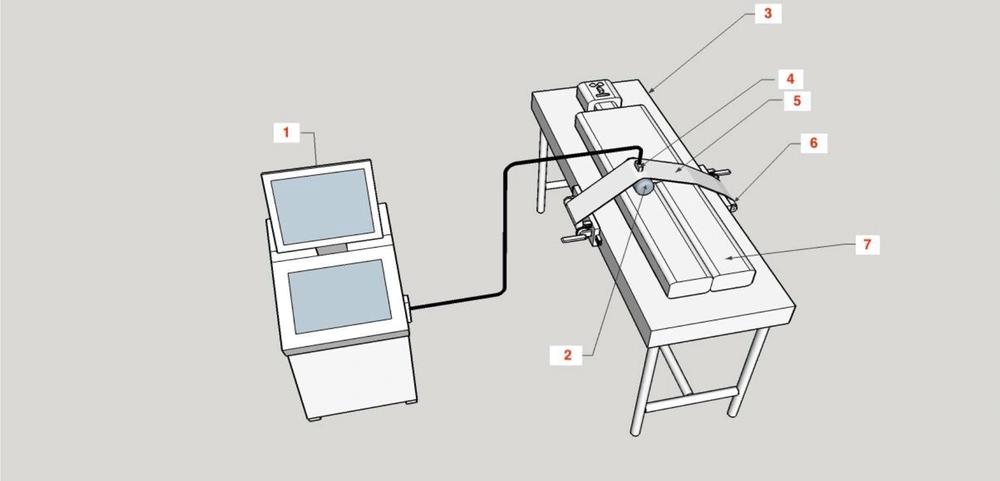
The device was created with a 20 cm wide compressor band made up of sonolucent plastic (translucent to ultrasound). The band was transversely placed on the groin area and fastened to two spools mounted on the bed rails on both sides (Figure 1).
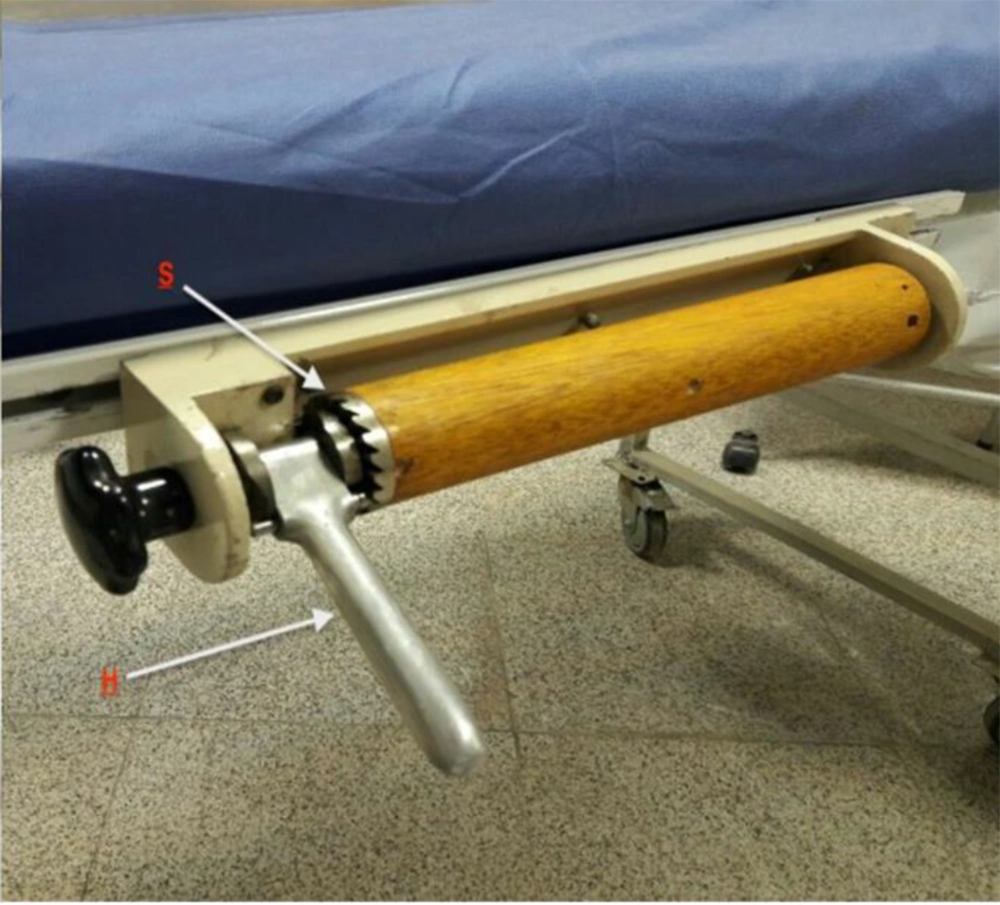
The blocks on which the spools were mounted could be moved up and down the bed to adjust for the patient’s height. Both spools could be wound and fixed with a ratchet mechanism so that the band pressure could be accurately adjusted and kept constant until unlocking the ratchet pawl (Figures 2 and 3).
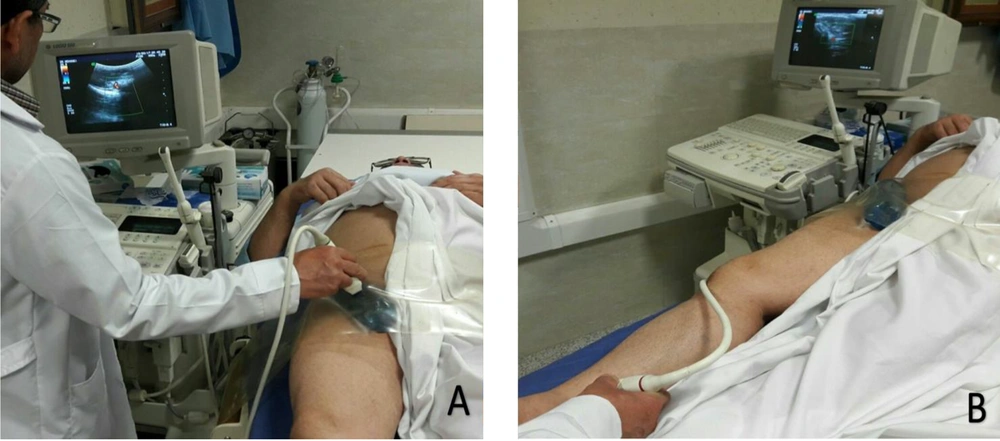
A fluid-containing pad was placed between the compressor band and the groin area to apply localized pressure on the patient’s groin (Figure 1). Ultrasound gel was applied to the skin, the pad, and between the pad and the band to control the status of arterial blood flow and the pseudoaneurysm using ultrasound during the compression. With the help of color Doppler ultrasound to monitor the area, the pressure applied by the device gradually increased until the blood flow in the pseudoaneurysm stopped, while blood flow still exited in the femoral vein. Upon reaching this state, the patient and the device were fixed and monitored for 20 minutes. During this period, the blood flow in the artery of the distal limb (tibialis posterior) was controlled using ultrasound (Figure 2).
3.5.2. Manual Ultrasound-Guided Compression
Femoral artery pseudoaneurysm was treated with manual ultrasound-guided compression for patients in the conventional compression group. In this technique, the ultrasound probe is placed on the groin to visualize the pseudoaneurysm directly. Pressure is applied to the probe to eliminate flow through the aneurysm neck while maintaining the arterial flow within the native femoral artery. The blockage of the pseudoaneurysm is monitored for 20 minutes. In case of failure, the procedure is repeated up to two more times, each for 20 minutes. All compression repairs were performed by the same expert radiologist with 15 years of experience.
3.6. Outcomes
The primary outcome of this study was comparing the frequency of complete recoveries between the two groups. The secondary outcome was comparing the groups' pain levels and short-term side effects.
3.7. Ethical Considerations
The research plan was fully explained to all patients. They completed and signed a written consent form before participating in the study. The patients' privacy was protected during the study, and their information was coded before transfer to the statistical software. The research proposal was approved by the Research Deputy (code: 951548) and the Ethics Committee of Mashhad University of Medical Sciences (IR.MUMS.MEDICAL.REC.1397.587). The patients were fully supported by the researchers in the event of any research-related complications and could withdraw from the research at any stage. The study was also registered at the Iranian Registry of Clinical Trials (IRCT20190701044059N1).
3.8. Statistical Analysis
Continuous variables were reported as mean ± standard deviation and categorical variables were reported as numbers (percentages). In the data analysis, the normality of the data was assessed using a one-sample Kolmogorov-Smirnov test with Lilliefors modification. All variables except age and BMI had non-normal distribution. The quantitative variables of the two groups were compared using the Independent-Sample t-test or its nonparametric equivalent, the Mann-Whitney test. The Pearson chi-Square test was used to compare the frequency distribution of qualitative data in the two groups. In cases where more than 20% of the expected frequencies were less than 5 (Cochran’s rule), Fisher’s exact test was used. The statistical software used in this study was IBM SPSS Statistics for Windows (IBM Corp. Released 2010. IBM SPSS Statistics for Windows, Version 19.0. Armonk, NY: IBM Corp.). In all tests, the level of statistical significance was P < 0.05.
4. Results
4.1. Background Information
This study included 22 patients (13 males/9 females) with a mean age of 65.5 ± 3.5 years. The patients were divided into a group receiving compression treatment with the device (n = 11) and a group receiving manual ultrasound-guided compression treatment (n = 11) (Figure 4). The two groups did not significantly differ in terms of demographic variables, including gender, age, and BMI, or pseudoaneurysm-related factors, including the time interval between angiography and the visit for pseudoaneurysm treatment, the dimensions and the neck size of the pseudoaneurysm, and the ratio of thrombosis to free lumen (Table 1). All patients in both groups were receiving anti-thrombotic medication.
| Variables | Compression with the invented device (N:11) | Manual compression; (N: 11) | P-value |
|---|---|---|---|
| Gender | 0.665 | ||
| Male | 7 (63.6) | 6 (54.5) | |
| Female | 4 (36.4) | 5 (45.5) | |
| Age (y) | 65.3 ± 3.7 | 65.7 ± 3.4 | 0.769 |
| BMI (kg/m2) | 27.8 ± 1.5 | 28.6 ± 1.4 | 0.191 |
| Patients receiving anticoagulant | 11 (100) | 11 (100) | - |
| Time between angiography and pseudoaneurysm treatment (hours) | 33.1 ± 12.2 | 30.0 ± 12.6 | 0.399 |
| Length of the pseudoaneurysm (mm) | 18.5 ± 4.2 | 21.6 ± 5.4 | 0.134 |
| Width of the pseudoaneurysm (mm) | 32.4 ± 4.2 | 34.6 ± 8.2 | 0.716 |
| Size of the pseudoaneurysm neck (mm) | 3.9 ± 1.6 | 4.3 ± 0.9 | 0.170 |
| Thrombosis to open lumen (%) | 14.6 ± 12.90 | 29.9 ± 15.1 | 0.346 |
a Values are expressed as mean ± SD or No. (%).
Abbreviation: BMI, Body Mass Index.
4.2. Outcomes of Interventions
The interventions and follow-ups were completely performed for all patients. There was no significant difference between the two groups regarding the duration of interventions (Table 2).
Of the 11 pseudoaneurysms in the device-assisted group, one (0.09%) was successfully occluded after the first attempt, eight (72%) required a second attempt to achieve complete thrombosis, and two (0.18%) were successfully occluded by the third attempt. However, in the manual compression group, all five (45.5%) successfully occluded pseudoaneurysms required a second compression attempt, while there was no success in the rest of the cases following the third compression attempt. The group of patients with device-assisted compression repair had lower pain intensity and higher treatment success rate (Table 2). None of the patients exhibited any side effects.
| Variables | Compression with the invented device (N:11) | Manual compression; (N: 11) | P-value |
|---|---|---|---|
| Duration of intervention (minutes) | 41.8 ± 10.7 | 40.0 ± 8.9 | 0.654 |
| Pain score during intervention | 5.0 ±1.0 | 6.0 ± 0.8 | 0.024 |
| Outcome | 0.004 | ||
| Success | 11 (100) | 5 (45.5) | |
| Failure | 0 (0) | 6 (55.5) | |
| Side effects | 0 (0) | 0 (0) | - |
a Values are expressed as mean ± SD or No. (%).
5. Discussion
In this study, we evaluated the efficacy of a compression device in treating angiography-induced FAP compared to conventional ultrasound-guided compression repair. The most important findings of this study were a lower pain score and a higher success rate observed using the new device for FAP compression repair.
The research outcome that was of greater interest was the success rate of the treatment. In this regard, the results showed that the device-assisted repair improved the success rate of FAP treatment by about 50% compared to conventional compression repair. All 11 patients treated with the invented device experienced a successful treatment. However, the success rate was about 45% in the conventional compression group. Although other studies have shown that manual ultrasound-guided compression can achieve a success rate of 90% (13), the majority of studies conducted since 1991, when Kus et al. introduced manual ultrasound-guided compression, have reported a success rate of 57% to 99% (14). Therefore, considering the workload and environmental stressors in a tertiary hospital, achieving a success rate of 45% for manual ultrasound-guided compression is not unexpected. This result highlights that the success rate of manual ultrasound-guided compression depends not only on patient- and disease-related parameters but also on therapist-related factors such as fatigue. Indeed, several studies have reported that manual compression may induce operator fatigue and reduce the accuracy and success rate of the procedure (2, 5). Using compression devices to apply pressure was intended to address this issue. Another explanation for the lower success rate of manual compression in the current study is that all our patients in both groups were receiving anti-thrombotic medication, which has been reported as a significant risk factor for ultrasound guided compression repair failure in the post-procedural period (15).
There is also little discussion in the literature regarding the number of attempts in compression repair. The only remarkable result in our study was that none of the pseudoaneurysms in the manual compression group was successfully occluded after the third attempt. One explanation is that the prolonged procedure makes it technically difficult to hold the probe at a similar location and produce constant compression.
Fellmeth et al. first attempted to use C-clamp assistance to hold the transducer in the optimal compression site and reduce operator fatigue. The authors reported a success rate of 71 to 92%. However, the mean time for the procedure was 59 minutes, during which the ultrasound unit could not be moved (16).
Trerotalo used FemoStop (Upsala, Sweden), a compression device, to treat pseudoaneurysms. Water was used to fill the device’s cushion to partially monitor the procedure using ultrasound (17). Later, Chatterjee et al. used FemoStop in a different method that did not require ultrasound monitoring, and the patient was allowed to return to the ward. The success rate in this study was 74%, about 25% lower than the rate achieved in our research, and it was more time-consuming, with a mean compression time of 33 minutes (18). The higher success rate of our device cannot be entirely attributed to its design, as factors such as the patient’s history, use of anticoagulants, and characteristics of the pseudoaneurysm may have also affected the success rate. These two studies are not comparable in all terms (2, 19, 20).
The advantages of our compression device include the ability to maintain constant localized pressure with a ratchet mechanism and direct ultrasound monitoring. However, its non-portability may be considered a drawback.
Another important outcome was the lower mean pain score in the group of device-assisted compression compared to the conventional compression group, with the mean pain scores of 5 and 6, respectively. In addition to constant pressure, using a gel-containing pad may create a cooling effect, which alleviates the patient’s pain during the procedure. Since the variable of interest is "pain," even a single-unit reduction can be clinically significant. However, it is essential to note that VAS, similar to other unidimensional pain scales, is a subjective method, and pain perception may significantly vary among individuals. To the best of our knowledge, no other clinical trial has compared the pain scores of FAP treatment interventions as an outcome. Hence, no references exist to compare the findings of this study. Therefore, the clinical significance of lower pain intensities in our study remains unclear. What is clear is that moderate to high pain scores remain one of the drawbacks in ultrasound-guided compression treatment, and even though the invented device reduced the pain score, the mean pain score was still 5 out of 10. Therefore, as suggested in other studies for conventional compression, the repair with the compression device should also include the administration of analgesics and sedatives as needed (5).
The patients in our study were also monitored for side effects, and no short-term side effect was observed in any patient. Likewise, other studies have reported no specific side effects associated with conventional compression but have mainly highlighted the limitations of ultrasound-guided compression, such as the length of the intervention and its labor intensiveness for sonographers (21).
Another essential feature that may influence the success rate of the treatment is the pseudoaneurysm neck size. Some previous studies have shown that aneurysm neck length is the only relevant prognostic factor for time to thrombosis (22), and the length < 10 mm is a statistically significant predictor of failure in ultrasound-guided compression repair (5). In our study, the mean pseudoaneurysm neck length was about 3 mm shorter in the device-assisted treatment group than in the control group. Although the success rate in the device-assisted treatment group was higher in the current study, the difference between pseudoaneurysm features was not statistically significant and, therefore, not comparable between the two groups.
It should be noted that there are other therapies available for FAP, although not all of them are as accepted as compression repair, primarily because of their potential complications. For surgical treatments, complications occur in about 20% of cases and include severe bleeding, infection, and even mortality. Although thrombin injection repair has a success rate of almost 90%, it also causes potential serious complications such as peripheral embolization (23). In addition, UGTI is not always successful on the first attempt and sometimes requires repeated injections or operations (5, 24).
Overall, a 100% therapeutic success rate and 0% side effects are significant outcomes that justify the use of this device in the ultrasound-guided compression repair of pseudoaneurysms. Although using a device in this procedure is not a novel idea, our device can be easily installed and used at any center with the minimum equipment. Therefore, it can be claimed that the compression device offers a simple solution for safer and more successful repair of pseudoaneurysms by eliminating the main limitation of manual ultrasound-guided compression, that is, physician fatigue and its impact on the proper procedure (2, 7, 25).
This study had several limitations. Although our sample size was similar to that of most other studies in this field, it is still considered small. We studied 22 patients, while many studies in the field are either case reports and case series (25, 26) or observational and interventional studies with sample sizes of fewer than 50 patients (2, 27, 28). Although some studies have sample sizes of more than 200 patients, they are relatively few in number (19, 29). The small sample size of most studies in this field can be attributed to the low prevalence of pseudoaneurysms. Thus, our first suggestion for future research is to gather a larger sample over a prolonged period of time with a multicentric design to increase the generalizability of findings. Another limitation was achieving desirable intervention blindness for patients and therapists. Hence, the use of the device may have affected the recorded data, although we attempted to minimize this impact by enlisting the help of a radiologist outside the research team. The use of VAS as a subjective pain scale is another important limitation, which may be of less value for making comparisons among individuals at the one time point. Further studies incorporating stimulus-response function tests for each patient (30) could provide more reliable comparisons of pain scores among individuals. Moreover, long-term follow-up exams could offer valuable insights into comparing pain scores and complications between groups.
In conclusion, the results of this study suggest that the proposed device is safe and may improve the success rate while reducing pain scores in ultrasound-guided compression repair as an alternative to manual compression treatment. To achieve better generalizability, it is necessary to conduct future studies with longer follow-ups, larger sample volumes, and comparisons with other conventional methods. Constructing and installing new equipment on the device, such as manometers, to apply controlled pressure is also suggested for future research.