1. Background
Short stature (SS) is defined as a height that is less than 2 standard deviations below the mean or below the third percentile for healthy children of a given age, sex, and race (1). It is one of the most common concerns for parents and pediatric physicians. Children with SS often exhibit slow growth, short stature, and other symptoms that can significantly impact their physical and mental development (2, 3). According to Chinese and World Health Organization (WHO) growth references, the age-standardized and age-gender-standardized prevalence of SS in China is as high as 3.70% and 2.69%, respectively, among 7 - 18-year-old school children (4).
The main causes of SS in children are idiopathic short stature (ISS) and growth hormone deficiency (GHD), accounting for 41% and 29% of all cases, respectively (5). The diagnosis of GHD primarily relies on growth hormone stimulation tests (GHSTs), while the diagnosis of ISS is made through exclusion, only after ruling out other diseases that can cause SS using auxology, biochemical, and imaging evaluations (6, 7). However, GHSTs can only be performed in a hospital setting and require patients to receive stimulants and have their blood drawn multiple times. Additionally, the false-positive rate of GHSTs is up to 15% (8, 9). Therefore, GHD is diagnosed only when two GHSTs are positive (8, 9). Given these challenges, a noninvasive and convenient method to distinguish GHD from ISS would be highly beneficial for pediatric physicians.
The pituitary gland (PG) is one of the most important and complex endocrine glands in the human body, regulating numerous hormone axes and surrounding endocrine organs. Previous studies have shown that the size and shape of the PG are closely related to the secretion of growth hormone (GH) (10, 11). Investigating changes in PG morphology in children may indirectly reflect GH secretion. Magnetic resonance imaging (MRI) is a non-invasive examination that can clearly show changes in PG morphology in children. It can improve the accuracy of etiological diagnoses in children with SS and reduce the number of invasive examinations and procedures. Pituitary MRI has a high resolution for soft tissues and can be used for multi-orientation and multi-parameter imaging. It not only shows subtle morphological and structural changes in the PG but also its signal intensity changes (12-14). The Growth Hormone Research Society (GRS) has recommended that PG height, volume, anatomy of the PG stalk, and the position of the posterior pituitary should be recorded from an MRI in confirmed GHD cases (15). However, there is still no systematic research on the correlation between PG morphology and GH levels in children with GHD or ISS. Therefore, we conducted a cross-sectional study of patients with GHD or ISS who underwent pituitary MRI at our institution from January 2020 to October 2022 to provide a reference for more accurate diagnoses of ISS and GHD.
2. Objectives
Growth hormone and insulin-like growth factor-I are the most effective therapeutic drugs for treating GHD, but their suitability for ISS remains controversial. Therefore, distinguishing between these two etiologies of SS is crucial. Currently, the diagnosis of GHD depends on GHSTs, while the diagnosis of ISS is achieved through exclusion, an invasive process. A noninvasive and convenient method to distinguish GHD from ISS would be highly valuable.
3. Patients and Methods
3.1. Participants and Groups
In this cross-sectional study, we enrolled patients aged 3 to 14 years who presented with SS and underwent GHSTs and PG MRI at our hospital from January 2020 to October 2022. The diagnoses of ISS or GHD were made according to the Consensus Statement on ISS and GHD (1, 15). The exclusion criteria were as follows: Poor image quality, incomplete image or clinical data, previous or current use of exogenous hormones, intracranial abnormalities such as space-occupying lesions or deformities, abnormal morphology or structure of the PG, anterior pituitary hormone deficiencies such as thyroid-stimulating hormone or adrenocorticotropic hormone deficiency, severe liver or kidney dysfunction, and malignant tumors.
A total of 205 patients were enrolled, comprising 83 with ISS and 122 with GHD. The GHD patients were divided into two groups based on the peak GH level in GHSTs: The absolute GHD (AGHD) group (GH < 5 µg/L, N = 40) and the partial GHD (PGHD) group (GH 5 - 10 µg/L, N = 82) (16, 17). This study was approved by the hospital ethics committee (No: 2023 - 136 - 01). All participants were informed of the examination procedures and precautions before the MRI examination and signed an informed consent form for the MRI.
3.2. Methods for Pituitary Gland MRI
All enrolled patients underwent routine physical examinations after admission, and GHSTs were conducted according to previously reported methods (17). Two stimulating drugs, insulin and levodopa, were used for GHSTs, and blood levels of GH were measured before the injection and at 30, 60, 90, and 120 minutes after the injection. Patient PG morphology was assessed using a 3.0 T MRI scanner with the following parameters: T1-weighted imaging (WI): Repetition time (TR) 550 ms, echo time (TE) 16 ms; T2WI: TR 3000 ms, TE 80 ms; matrix: 216 × 198; slice thickness: Two mm; field of view (FOV): One hundred-sixty mm × 160 mm; and number of excitations: Two. After scanning, images were reviewed by highly qualified radiologists, each with more than five years of experience. Two authors independently recorded the features (PG height, length, width, volume, pituitary stalk diameter, pituitary stalk length, and pituitary morphology) and discussed their assessments with the corresponding author to reach a consensus if their initial evaluations differed.
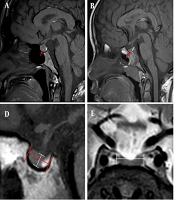
The morphology of the PG was categorized into three types: Convex (Figure 1A), flat (Figure 1B), and concave (Figure 1C). Additionally, PG height, length, pituitary stalk diameter, and pituitary stalk length were measured on sagittal T1 images (Figure 1D and F), and PG width was measured on the coronal T2 image (Figure 1E). According to the applicable ellipsoidal formula, the volume of the PG was calculated as (length × width × height)/2 (18). Bone age was estimated from X-rays of the left wrist by observing the morphology, size, and degree of development of the ossification centers in the left metacarpophalangeal bone, wrist bone, and the lower end of the radius and ulna.
The morphology and assessment of the pituitary gland (PG). A, Convex type; B, Flat type; C, Concave type; D, The height and length of the PG; E, The width of the PG; F, The diameter and length of the pituitary stalk; A, B, C, D, and F images are sagittal T1 weighted imaging, while E image is coronal T2 weighted imaging.
3.3. Statistical Analyses
The SPSS 19.0 software (IBM SPSS Statistics for Windows, Version 19.0. Armonk, NY: IBM Corp) was used for all statistical analyses. Quantitative data were expressed as mean ± standard deviation (SD), and comparisons between two groups were made using two independent samples t-tests. Categorical data were expressed as No. (%), and comparisons between groups were made using the chi-square (χ²) test. Additionally, Pearson’s linear correlation was used to analyze the correlation between PG features and GH levels, represented by r, which ranges from -1 to 1. An r value of -1 indicates a perfect negative correlation, +1 indicates a perfect positive correlation, and 0 indicates no linear correlation. The closer r is to -1 or +1, the stronger the linear relationship between the two variables.
Receiver operating characteristic (ROC) curves were used to evaluate the sensitivity and specificity of PG features in diagnosing ISS and GHD. The cut-off value was obtained by calculating the Youden Index (sensitivity + specificity - 1), with the diagnostic threshold corresponding to the highest Youden index considered the optimal cut-off value. The area under the ROC curve (AUC) was used to measure diagnostic accuracy; the AUC ranges between 0 and 1, with higher values indicating greater accuracy. A P-value of < 0.05 was considered statistically significant.
4. Results
4.1. Patient Enrollment and Baseline Clinical Characteristics
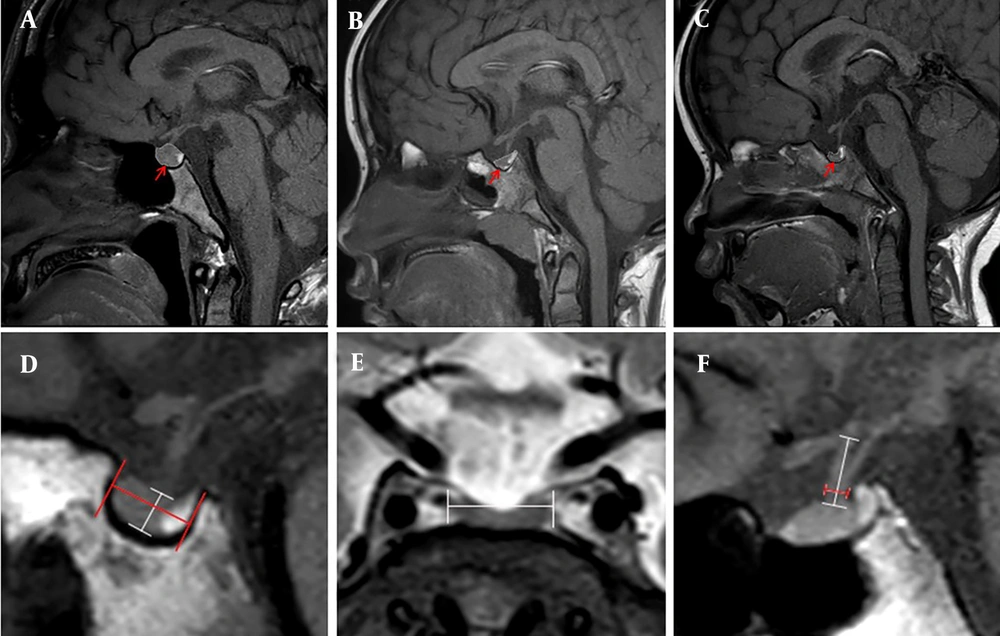
Of the 827 patients initially admitted with SS, 378 were excluded due to either not undergoing GHSTs or having unsuccessful GHSTs, 181 were excluded because no MRI images were available, and 63 were excluded due to brain abnormalities or other known causes of SS. Ultimately, 205 patients were included in the study, comprising 83 patients with ISS and 122 patients with GHD. Among the GHD patients, 40 had AGHD and 82 had PGHD, as shown in Figure 2. The baseline clinical characteristics of patients with ISS and GHD are presented in Table 1. There were no significant differences between the GHD and ISS patients regarding age, sex, percentage of full-term and vaginal delivery, birth weight and height, bone age, or abnormal bone age (all P > 0.05). However, the GH peak in GHST was significantly lower in the GHD group than in the ISS group (P < 0.05).
Flowchart of patient enrollment GHSTs. Abbreviations: GHSTs, growth hormone stimulation tests; PG, pituitary gland; MRI, magnetic resonance imaging; ISS, idiopathic short stature; GHD, growth hormone deficiency; AGHD, absolute growth hormone deficiency; PGHD, partial growth hormone deficiency.
| Variables | ISS; (N = 83) | GHD; (N = 122) | t/χ2 | P-value |
|---|---|---|---|---|
| Age (y) | 7.93 ± 2.92 | 7.72 ± 2.84 | 5.188 | 0.922 |
| Male | 55 (66.26) | 81 (66.39) | 0.001 | 0.985 |
| Full-term | 75 (90.36) | 114 (93.44) | 0.652 | 0.420 |
| Vaginal delivery | 34 (40.96) | 43 (35.25) | 0.689 | 0.407 |
| Intrauterine hypoxia | 5 (6.02) | 6 (4.92) | 0.119 | 0.730 |
| Birth weight (kg) | 3.14 ± 0.65 | 3.03 ± 0.44 | -1.421 | 0.157 |
| Birth height (cm) | 49.11 ± 5.66 | 49.30 ± 2.08 | 0.345 | 0.731 |
| Bone age (y) | 6.07 ± 1.92 | 5.82 ± 2.05 | 6.734 | 0.346 |
| Abnormal bone age (y) | 1.86 ± 1.86 | 1.90 ± 1.69 | 0.185 | 0.854 |
| GH peak (ng/mL) | 15.17 ± 4.82 | 6.23 ± 2.53 | -17.270 | 0.001 |
Abbreviations: ISS, idiopathic short stature; GHD, growth hormone deficiency; GH, growth hormone.
a Values are expressed as mean ± SD or No. (%).
b Abnormal bone age: chronological age minus bone age.
4.2. Morphological Differences in Pituitary Gland Between Growth Hormone Deficiency and Idiopathic Short Stature Patients
As shown in Table 2, pituitary stalk diameter, PG height, PG volume, and dorsum sellae were significantly lower, while the tuberculum sellae were higher in the GHD group compared to the ISS group (all P < 0.05). However, there were no significant differences in pituitary stalk length, PG length, PG width, cavernous sinus width, optic chiasma height, or optic chiasma width between the two groups (all P > 0.05). Regarding morphology type, the proportion of concave PG was higher, and the proportion of flat PG was lower in the GHD group (all P < 0.05), but there was no difference in convex PG between the two groups (all P > 0.05).
| Variables | ISS; (N = 83) | GHD; (N = 122) | t/χ2 | P-value |
|---|---|---|---|---|
| Pituitary stalk diameter (mm) | 2.30 ± 0.36 | 2.10 ± 0.44 | -3.465 | 0.001 |
| Pituitary stalk length (mm) | 7.24 ± 1.37 | 7.37 ± 1.44 | 0.658 | 0.511 |
| PG length (mm) | 8.89 ± 0.98 | 8.73 ± 0.95 | -1.158 | 0.248 |
| PG width (mm) | 11.95 ± 1.23 | 11.75 ± 1.20 | -1.203 | 0.231 |
| PG height (mm) | 5.96 ± 0.92 | 4.77 ± 0.81 | -9.498 | 0.001 |
| PG volume (mm³) | 311.19 ± 34.70 | 242.74 ± 45.11 | -11.671 | 0.001 |
| Tuberculum sellae (mm) | 8.42 ± 1.26 | 8.87 ± 1.76 | 2.000 | 0.047 |
| Dorsum sellae (mm) | 6.30 ± 1.16 | 5.72 ± 1.23 | -3.385 | 0.01 |
| Cavernous sinus width (mm) | 29.06 ± 2.68 | 28.96 ± 2.53 | -0.265 | 0.791 |
| Optic chiasma height (mm) | 3.71 ± 0.82 | 3.57 ± 0.75 | -1.289 | 0.199 |
| Optic chiasma width (mm) | 12.71 ± 1.52 | 12.24 ± 1.53 | -0.019 | 0.985 |
| Morphology type | 13.111 | 0.001 | ||
| Concave type | 10 (12.05) | 32 (26.23) c | ||
| Flat type | 45 (54.22) | 48 (39.34) c | ||
| Convex type | 28 (33.73) | 42 (34.43) |
Abbreviations: PG, pituitary gland; ISS, idiopathic short stature; GHD, growth hormone deficiency.
a Values are expressed as mean ± SD or No. (%).
b The volume of the PG is defined as (length × width × height)/2.
c P < 0.05.
4.3. Comparison of Baseline Clinical Characteristics and Pituitary Gland Morphology Between Patients with Absolute Growth Hormone Deficiency and partial Growth Hormone Deficiency
Patients with GHD were divided into two groups based on the peak level of GH in the GHSTs: An AGHD group (N = 40) and a PGHD group (N = 82). Their baseline clinical characteristics and PG morphology features are presented in Tables 3 and 4. There were no significant differences in baseline clinical characteristics between the two groups (all P > 0.05), except for the GH peak in GHSTs (P < 0.05), as shown in Table 3. Regarding PG morphology, PG height and PG volume were significantly lower in the AGHD group compared to the PGHD group (both P < 0.05), as shown in Table 4.
| Variables | AGHD; (N = 40) | PGHD; (N = 82) | t/χ2 | P-value |
|---|---|---|---|---|
| Age (y) | 8.07 ± 2.56 | 7.55 ± 2.97 | 1.012 | 0.314 |
| Male | 25 (62.50) | 56 (68.29) | 0.404 | 0.525 |
| Full-term | 39 (97.50) | 75.(91.46) | 1.599 | 0.206 |
| Vaginal delivery | 13 (32.50) | 30 (36.59) | 0.197 | 0.657 |
| Intrauterine hypoxia | 3 (7.50) | 3 (3.66) | 0.848 | 0.357 |
| Birth weight (kg) | 3.02 ± 0.49 | 3.04 ± 0.42 | -0.168 | 0.867 |
| Birth height (cm) | 49.13 ± 1.94 | 49.39 ± 2.15 | -0.685 | 0.495 |
| Bone age (y) | 6.20 ± 1.77 | 5.63 ± 2.16 | 1.435 | 0.154 |
| Abnormal bone age (y) | 1.88 ± 1.47 | 1.91 ± 1.79 | -0.130 | 0.897 |
| GH peak (ng/mL) | 3.19 ± 1.11 | 7.72 ± 1.48 | -17.150 | 0.001 |
Abbreviations: GH, growth hormone; AGHD, absolute growth hormone deficiency; PGHD, partial growth hormone deficiency.
a Values are expressed as mean ± SD or No. (%).
b Abnormal bone age: chronological age minus bone age.
| Variables | AGHD; (N = 40) | PGHD; (N = 82) | t/χ2 | P-value |
|---|---|---|---|---|
| Pituitary stalk diameter (mm) | 2.12 ± 0.45 | 2.08 ± 0.43 | 0.485 | 0.629 |
| Pituitary stalk length (mm) | 7.55 ± 1.48 | 7.29 ± 1.42 | 0.927 | 0.357 |
| PG length (mm) | 8.72 ± 0.95 | 8.73 ± 0.96 | -0.084 | 0.933 |
| PG width (mm) | 11.50 ± 0.95 | 11.87 ± 1.29 | -1.797 | 0.075 |
| PG height (mm) | 4.21 ± 1.01 | 5.05 ± 0.51 | -6.063 | 0.001 |
| PG volume (mm³) | 210.45 ± 55.90 | 258.49 ± 27.74 | -6.358 | 0.001 |
| Tuberculum sellae (mm) | 8.84 ± 1.60 | 8.89 ± 1.85 | -0.147 | 0.883 |
| Dorsum sellae (mm) | 5.73 ± 1.02 | 5.72 ± 1.32 | 0.040 | 0.968 |
| Cavernous sinus width (mm) | 28.71 ± 2.46 | 29.08 ± 2.57 | -0.755 | 0.453 |
| Optic chiasma height (mm) | 3.51 ± 0.60 | 3.59 ± 0.82 | -0.605 | 0.547 |
| Optic chiasma width (mm) | 12.54 ± 1.42 | 12.79 ± 1.55 | -0.897 | 0.372 |
| Morphology type | 15.771 | 0.003 | ||
| Concave type | 17 (42.50) | 23 (28.05) b | ||
| Flat type | 14 (35.00) | 31 (37.80) b | ||
| Convex type | 9 (22.50) | 28 (34.15) |
Abbreviations: PG: pituitary gland; AGHD: absolute growth hormone deficiency; PGHD: partial growth hormone deficiency.
a Values are expressed as mean ± SD or No. (%).
b P < 0.05.
4.4. The Correlation Between Pituitary Gland Height, Pituitary Gland Volume, and Growth Hormone Peak Level
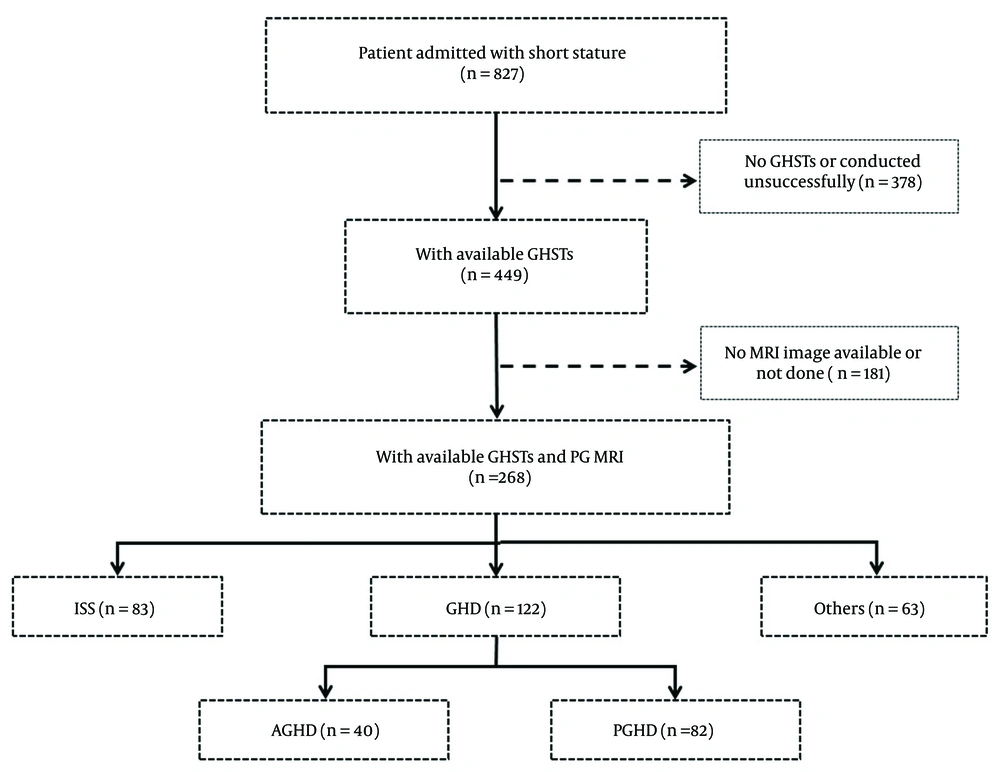
To investigate the correlations between PG morphology and GH peak level, we performed Pearson’s linear correlation analysis on pituitary stalk diameter, PG height, PG volume, tuberculum sellae, and dorsum sellae. The results showed that PG height (r = 0.635, P < 0.01) and PG volume (r = 0.786, P < 0.01) were positively correlated with GH peak level, both in the ISS group (height: r = 0.285, P < 0.01; volume: r = 0.644, P < 0.01) and the GHD group (height: r = 0.553, P < 0.01; volume: r = 0.686, P < 0.01), as shown in Figure 3. However, pituitary stalk diameter (r = 0.193, P = 0.012), tuberculum sellae (r = -0.097, P = 0.169), and dorsum sellae (r = 0.181, P < 0.01) showed relatively poor correlation with GH peak level, both in the ISS group (pituitary stalk diameter: r = 0.002, P = 0.988; tuberculum sellae: r = 0.004, P = 0.974; dorsum sellae: r = -0.038, P = 0.735) and the GHD group (pituitary stalk diameter: r = -0.091, P = 0.317; tuberculum sellae: r = 0.033, P = 0.717; dorsum sellae: r = -0.035, P = 0.705).
The correlation between PG height, PG volume and GH peak level. A, The correlation between PG height and GH peak level in all patients; B, The correlation between PG height and GH peak level in the ISS group; C, The correlation between PG height and GH peak level in the GHD group; D, The correlation between PG volume and GH peak level for all patients; E, The correlation between PG volume and GH peak level in the ISS group; F, The correlation between PG volume and GH peak level in the GHD group. Abbreviations: PG, pituitary gland; ISS, idiopathic short stature; GHD, growth hormone deficiency; GH, growth hormone.
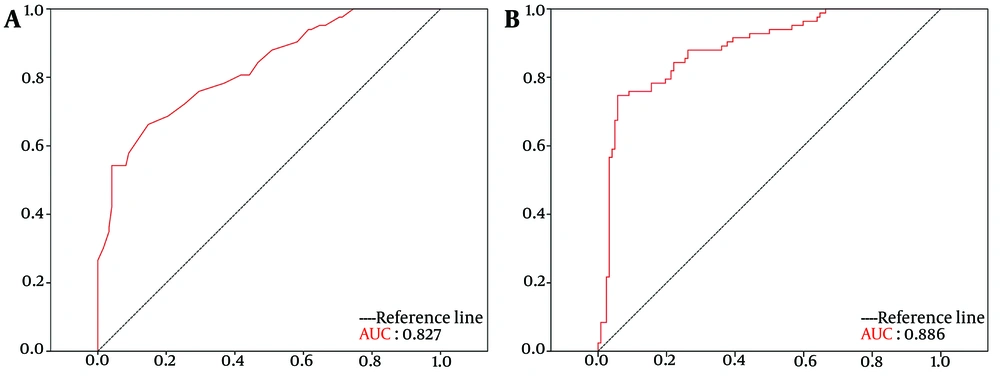
4.5. Receiver Operating Characteristic Analysis of Pituitary Gland Height and Pituitary Gland Volume
To determine the diagnostic accuracy of PG height and PG volume, we conducted ROC curve analysis, and the AUC was calculated. The results showed that both PG height and PG volume were significantly affected by GHD (Figure 4). Specifically, PG height, with a cut-off value of 5.55 mm, had a sensitivity of 66.3%, a specificity of 85.2%, a positive predictive value of 81.75%, and a negative predictive value of 71.66% (AUC = 0.827 [95% CI: 0.770 – 0.884], P < 0.001). Similarly, PG volume, with a cut-off value of 276.74 mm³, had a sensitivity of 84.3%, a specificity of 77.9%, a positive predictive value of 79.23%, and a negative predictive value of 83.23% (AUC = 0.886 [95% CI: 0.839 – 0.933], P < 0.001).
5. Discussion
To the best of our knowledge, this is the first attempt to find a noninvasive and convenient method to help pediatric physicians differentiate ISS from GHD. In this study, we found that both PG height and PG volume were positively correlated with GH peak levels in both ISS and GHD patients, making them promising markers for identifying ISS and GHD. Additionally, both markers demonstrated high sensitivity (PG height: 66.3%; PG volume: 84.3%) and specificity (PG height: 85.2%; PG volume: 77.9%). In terms of PG morphology, a concave PG was more common in patients with GHD, while a flat PG was more common in patients with ISS. However, the overall utility of PG morphology in diagnosing ISS and GHD still requires further evaluation.
The pituitary is an ellipsoid organ located in the sella turcica at the base of the skull, consisting of the adenohypophysis and the neurohypophysis. The pituitary stalk connects the hypothalamus to the pituitary, transmitting regulatory hormones from the hypothalamus to the anterior pituitary through the portal system and regulating the synthesis and secretion of GH via the anterior pituitary GH cells (19). The morphology and function of the pituitary are closely linked to GH secretion, which is itself closely associated with human growth and development. Insufficient secretion of GH affects bone growth, cardiovascular function, and metabolism in children, thereby impacting their quality of life (20, 21). Magnetic resonance imaging of the pituitary can clearly reveal the anatomical relationship between the pituitary and its surrounding structures and allows clinicians to observe both the normal anatomical structure and any pathological changes in the pituitary gland from multiple perspectives (13, 22).
The morphological characteristics of the pituitary gland are closely related to its function. In particular, the coronal and sagittal heights of the pituitary are positively correlated with the peak value of GH, and the height of the pituitary can effectively reflect the development of the anterior pituitary. When blood levels of GH are below normal, the synthesis and secretion of GH can be increased through negative feedback regulation. Long-term insufficient secretion of GH can, to some extent, lead to reactive hyperplasia and hypertrophy of the pituitary gland. Therefore, the height and volume of the pituitary gland are closely related to the synthesis and secretion of GH. Excessive secretion of GH can lead to pituitary hyperplasia or tumors, while insufficient secretion can result in SS (11, 23). Furthermore, research indicates that pituitary size is a useful marker in diagnosing GHD in cases of SS (24). Compared to healthy individuals, the pituitary volume and height in children with GHD decrease more than in those with ISS (25, 26). In our study, we also found that pituitary height and volume are closely related to GH secretion and that pituitary height and volume were significantly lower in GHD patients compared to ISS patients. More importantly, pituitary height and volume were found to be promising markers for identifying ISS and GHD, both demonstrating high sensitivity (PG height: 66.3%; PG volume: 84.3%) and specificity (PG height: 85.2%; PG volume: 77.9%).
The morphology of the pituitary gland can be classified into three types: Concave, flat, and convex, with the flat type being the most common. In patients with GHD, the development of the anterior pituitary gland is typically poor, leading to decreased function of the adenohypophysis and consequently reduced GH levels. Furthermore, in these patients, the height and volume of the pituitary gland are smaller, and the proportion of the concave type is higher (27). In this study, we found that the proportion of the concave type was higher in patients with GHD than in those with ISS, suggesting that pituitary function was indeed poorer in patients with GHD.
Even so, our study has several limitations. First, this is a retrospective study, which may have biased the results. Second, although our study enrolled more cases than previous studies, observations of the sellar region structure, SS type, and clinical characteristics were limited. Therefore, it is necessary to conduct large-scale multicenter clinical studies to determine the predictive value of PG height and volume more robustly. Finally, a prediction model that combines clinical characteristics and PG morphology is needed, as it would be more helpful for pediatric physicians.
In summary, the volume, height, and shape of the PG are closely related to GH secretion and the etiology of SS. If the height of the PG is greater than 5.55 mm, the volume is greater than 276.74 mm³, and the morphology of the PG is flat or convex, ISS is more likely than GHD. With the advancement of MRI structural imaging and artificial intelligence, we will use high-resolution 3D structural imaging and big data analysis to further explore the morphological differences of the PG in patients with GHD and ISS, providing a potential non-invasive biomarker for the diagnosis and differential diagnosis of these two conditions in clinical practice.
In conclusion, we found that PG height, PG volume, and PG concave type can be used to differentiate GHD from ISS, which may be useful in the differential diagnosis of SS in children.