1. Background
Endometrial cancer is the most common gynecological cancer in economically developed regions and is associated with factors such as infertility, diabetes, polycystic ovary syndrome, and prolonged use of exogenous estrogen (1). In recent years, the incidence of endometrial cancer has been increasing, with a rising trend among younger individuals (1, 2). It ranks as the second most prevalent gynecological malignancy in China (3).
The recently revised Federation International of Gynecology and Obstetrics (FIGO) staging system for endometrial cancer in 2023 incorporates histopathological features based on the 2009 surgical staging. This enhancement aids gynecologists in tailoring treatment plans to improve patient prognosis and survival outcomes (4). Risk factors for poor prognosis include lymphovascular space invasion, high-grade histology, deep myometrial invasion, and cervical stromal invasion (5, 6).
Accurate magnetic resonance imaging (MRI) staging for cervical stromal invasion presents certain challenges (4, 7, 8). Clinically, extensive tumor spread into stromal tissue is readily detected, yet when a tumor infiltrates the cervical canal, making the interface between the tumor and cervix indistinct, evaluating stromal invasion becomes challenging (9). Cervical invasion is diagnosed when images reveal an interruption of the enhancing epithelium on dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) (10). The European Society of Urogenital Radiology recommends a delayed DCE-MRI phase (4 - 5 minutes post-contrast injection) for detecting cervical stromal invasion (11). However, literature indicates that cervical mucosal enhancement occurs earlier than stromal enhancement after contrast injection (12), suggesting that the arterial phase of DCE-MRI may provide added value in predicting cervical stromal invasion.
Given the increasing number of patients, optimizing time efficiency is a necessary consideration. Thus, this study utilized multiphase DCE-MRI scans to compare the diagnostic efficacy of the arterial phase (30 - 40 seconds post-injection) and delayed phase (4 - 5 minutes post-injection) in identifying cervical stromal invasion and to evaluate the additional value provided by the arterial phase.
2. Objectives
To assess and compare the diagnostic accuracy of DCE-MRI in the arterial phase and delayed phase for detecting cervical stromal invasion in cases of endometrial carcinoma.
3. Patients and Methods
3.1. Patients
This study was approved by the Ethics Committee of Beijing Obstetrics and Gynecology Hospital, Capital Medical University (approval: 2023-KY-020-01). Imaging and pathological data for patients with endometrial cancer, as defined by the 2009 FIGO staging criteria (13), who underwent surgical treatment in the Department of Gynecology and Obstetrics at Beijing Obstetrics and Gynecology Hospital, Capital Medical University, were retrospectively collected from January 2016 to December 2022. Cases were identified through the picture archiving and communication system (PACS), overseen by an image manager. The inclusion criteria were as follows: (1) MRI plain and dynamic enhanced scans were performed before hysterectomy, with complete imaging data available; (2) absence of MRI examination contraindications. Exclusion criteria included: Patients who had undergone radiotherapy, chemotherapy, uterine artery embolization, or other treatments before the MRI examination. The sample size for assessing cervical stromal invasion in endometrial cancer by MRI was calculated based on the diagnostic test sample size calculation formula.
3.2. Magnetic Resonance Imaging Examination Method
The patient's pelvic images were acquired using a 3.0 T MR scanner with an 8-channel phased-array coil. T1-weighted and diffusion-weighted imaging (DWI) included sagittal and axial views, while T2-weighted images were captured in coronal, sagittal, and axial views. Dynamic contrast-enhanced magnetic resonance imaging was performed in the sagittal plane, with a total of 6-phase enhancement images acquired over a 2-minute 15-second scanning duration. The delayed phase was scanned between 4 to 5 minutes. Gadodiamide (GE Pharmaceutical Co., Ltd, 287 mg/mL) was administered as the contrast agent, with an injection rate of 2.0 - 2.5 mL/s and a dose of 0.1 mmol/kg body weight.
3.3. Magnetic Resonance Image Analysis
The MR images for each patient were jointly analyzed by two radiologists in our hospital's radiology department. Consensus was reached after discussion, and in cases of discrepancy, assistance was sought from a senior radiologist. The radiologists were blinded to the final histopathological results.
The criteria for diagnosing cervical stromal invasion on DCE-MRI were as follows: (1) interruption of the normally enhanced cervical mucosa during the arterial phase (14); and (2) destruction of the normally enhanced cervical stroma by a low-intensity tumor during the delayed phase (11).
3.4. Statistical Method
Data analysis was performed using SPSS statistical software (SPSS Inc. Released 2008. SPSS Statistics for Windows, Version 17.0. Chicago: SPSS Inc.). Depending on data normality, continuous variables were presented as mean ± standard deviation (SD) or as median values, while categorical variables were expressed as percentages (%). The accuracy, sensitivity, specificity, positive predictive value, and negative predictive value of DCE-MRI in evaluating cervical stromal invasion in the arterial and delayed phases were calculated separately.
The McNemar test was applied to assess any statistical differences in sensitivity and specificity between the arterial and delayed phases for detecting cervical stromal invasion on DCE-MRI. Multiple logistic regression analysis was conducted to evaluate factors influencing the diagnosis of cervical stromal invasion in endometrial carcinoma on DCE-MRI, including variables such as tumor location, cervical polyps, and Nabothian cysts. Odds ratios (OR) and regression coefficients (β) were calculated, with a significance threshold of P < 0.05.
4. Results
4.1. Treatment of Patients
A total of 445 patients with endometrial cancer underwent surgical treatment and had complete MRI data available for analysis. Among these patients, 63 underwent hysterectomy alone; 153 underwent hysterectomy combined with bilateral salpingo-oophorectomy; 95 underwent hysterectomy with bilateral salpingo-oophorectomy and pelvic lymphadenectomy; 103 patients received hysterectomy, bilateral salpingo-oophorectomy, and both retroperitoneal and pelvic lymphadenectomy. Additionally, 31 patients received preoperative chemotherapy, followed by hysterectomy, bilateral salpingo-oophorectomy, and retroperitoneal and pelvic lymphadenectomy. Postoperative treatment included adjuvant radiotherapy for 96 patients, and concurrent radiotherapy and chemotherapy for 89 patients. The general characteristics of the patients are presented in Table 1.
| Characteristics | Values |
|---|---|
| Age (y) | 53.5 ± 3.1 |
| Number of women | 445 |
| Perimenopause | 187 (42) |
| Menopause | 258 (58) |
| BMI (kg/m2) | |
| < 18.5 | 12 (2.7) |
| 18.5 - 24.9 | 120 (27.0) |
| 25 - 29.9 | 237 (53.2) |
| ≥ 30 | 76 (17.1) |
| Stage b | |
| Ia | 227 (51.0) |
| Ib | 89 (20.0) |
| II | 71 (16.0) |
| III | 40 (9.0) |
| IV | 18 (4.0) |
| Tumor type b | |
| Endometrioid carcinoma | 371 (83.4) |
| Serous carcinoma | 37 (8.3) |
| Clear cell carcinoma | 13 (3.0) |
| Others | 24 (5.3) |
| Degree of tumor differentiation b | |
| Well-differentiated | 270 (60.6) |
| Moderately differentiated | 95 (21.3) |
| Poorly differentiated | 80 (18.1) |
a Values are expressed as mean ± SD or No. (%).
b Based on the 2009 FIGO staging standards (11).
4.2. Cervical Enhancement Characteristics on Dynamic Contrast-Enhanced Magnetic Resonance Imaging
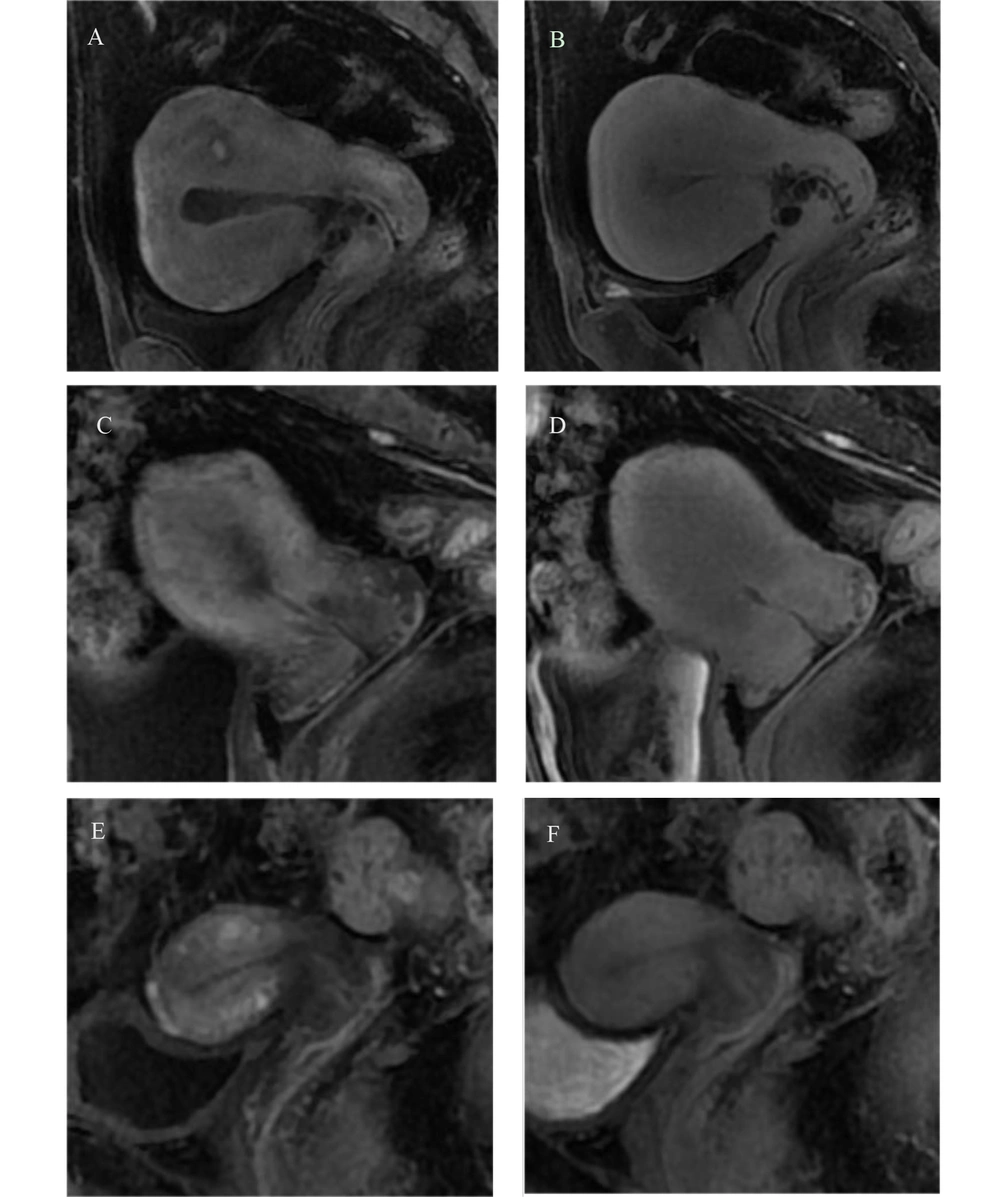
The cervical mucosa exhibited continuous enhancement in both the arterial and delayed phases on DCE-MRI. The enhancement of the cervical stroma was primarily observed in three patterns, as illustrated in Figure 1.
Uterine image in arterial phase and delayed phase of dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI). A, C, E, arterial phase images; B, D, F, delayed phase images. Cervical mucosa was significantly enhanced in A-F. Cervical mucosa with Nabothian cyst in A-D. The cervical stroma and the muscular layer of the uterine body were simultaneously enhanced, and the cervical stroma was significantly enhanced in the arterial phase in A and B. The enhancement degree of cervical stroma in the arterial phase was lower than that in the myometrium, and the enhancement degree of cervical stroma in the delayed phase was the same as that in the myometrium in C and D. The enhancement degree of cervical stroma in the arterial phase was lower than that in the uterine muscle layer, and the degree of enhancement in the delayed phase was still lower than that in the uterine muscle layer in E and F.
First, the cervical stroma and uterine muscle layer were enhanced simultaneously, with consistent enhancement across all stages, occurring in 31.4% of cases. Second, the cervical stroma showed a lower degree of enhancement in the arterial phase compared to the uterine muscle layer, but was similar to the uterine muscle layer in the delayed phase, observed in 34.2% of cases. Third, the cervical stroma had lower enhancement in the arterial phase relative to the uterine muscle layer, and this reduced enhancement persisted in the delayed phase, with an incidence of 34.4%.
4.3. Determination of Endometrial Cancer with Cervical Stromal Invasion by Dynamic Contrast-Enhanced Magnetic Resonance Imaging in the Arterial Phase and Delayed Phase Versus Surgical Pathologic Results
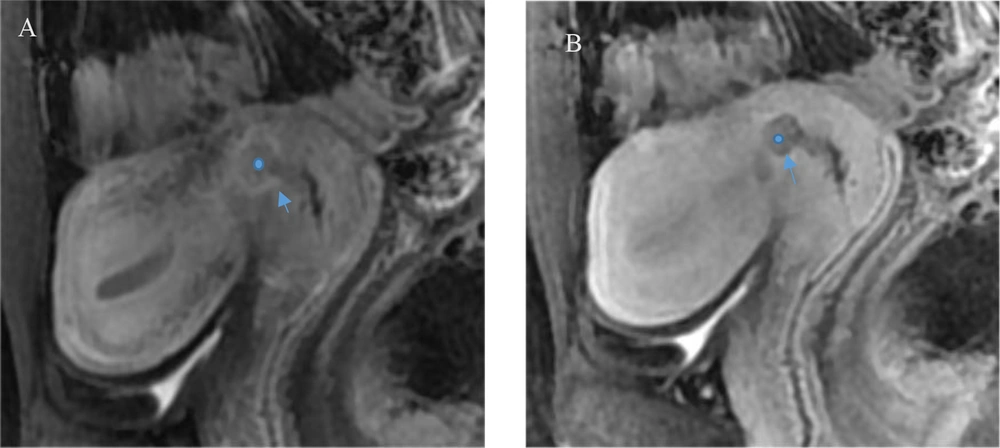
Among the 445 patients with endometrial cancer, a total of 107 patients were found to have cervical stromal invasion on pathology (Figure 2), with 71 cases classified as stage II, 29 cases as stage III, and 7 cases as stage IV. In the arterial phase of DCE-MRI, 30 patients with stage Ia and 11 patients with stage Ib were misdiagnosed as stage II. Additionally, 31 patients with stage II were misclassified as stage I, and 5 patients with stage II were diagnosed as having cervical cancer.
A 36-year-old patient with cervical stromal invasion of endometrial carcinoma. In the arterial phase, the tumor infiltrated the cervical stroma (circle) with unclear borders, and significant enhancement of the tumor border effect (arrow) is seen in the aera of junction with the cervix, and the enhancement of cervical mucosal is incomplete in A. In the delay period, the tumor infiltrated the cervical stroma (circle), with clear boundary (arrow), and the low intensity tumor destroyed the normal enhancement of the cervical stroma in B.
In the delayed phase, 28 patients with stage Ia and 12 patients with stage Ib were misdiagnosed as stage II. Furthermore, 25 patients with stage II were misclassified as stage I, and 8 patients with stage II were diagnosed as cervical cancer. The diagnostic results of DCE-MRI in both the arterial and delayed phases are presented in Table 2.
| Variable | Arterial phase | Delayed phase | ||
|---|---|---|---|---|
| + | - | + | - | |
| Pathology | ||||
| + | 71 | 36 | 74 | 33 |
| - | 41 | 297 | 40 | 298 |
The sensitivity, specificity, accuracy, positive predictive value, and negative predictive value of DCE - MRI for detecting cervical stromal invasion were as follows: For the arterial phase, the sensitivity was 66.4% [95% confidence interval (CI): 60.0 - 74.6%], specificity was 87.9% (95% CI: 83.9 - 91.0%), accuracy was 82.7% (95% CI: 78.9 - 85.9.1%), positive predictive value was 63.4% (95% CI: 54.1 - 71.7%), and negative predictive value was 89.2% (95% CI: 85.4 - 92.1%). For the delayed phase, the sensitivity was 69.1% (95% CI: 60.0 - 77.1%), specificity was 88.2% (95% CI: 84.3 - 91.2%), accuracy was 83.6% (95% CI: 80.0 - 86.8%), positive predictive value was 64.9% (95% CI: 55.8 - 73.1%), and negative predictive value was 90.0% (95% CI: 86.3 - 92.8%). The McNemar test was used to compare sensitivities and specificities between the arterial and delayed phases, yielding non-significant P-values (P = 1.00), indicating no statistically significant difference between the two phases in terms of sensitivity and specificity. Multiple logistic regression analysis identified two significant risk factors for misdiagnosis or missed diagnosis of cervical stromal invasion on DCE - MRI: Cancer located in the lower uterine segment or the internal os of the cervix, with an OR of 8.1 (95% CI: 2.6 - 25.5), β = 2.2, P < 0.01, and the presence of clustered cysts in the cervical mucosa, with an OR of 4.1 (95% CI: 1.5 - 11.4), β = 1.4, P = 0.01. The detailed sensitivity, specificity, accuracy, positive predictive value, and negative predictive value for radiologists diagnosing cervical stromal invasion in both arterial and delayed phases of DCE-MRI are provided in Table 3.
| Variables | Sensitivity (%) | Specificity (%) | Accuracy (%) | Positive predictive value (%) | Negative predictive value (%) |
|---|---|---|---|---|---|
| Arterial phase | 66.4 (71/107) | 87.9 (297/338) | 82.7 (368/445) | 63.4 (71/112) | 89.2 (297/333) |
| Delayed phase | 69.1 (74/107) | 88.2 (298/338) | 83.6 (372/445) | 64.9 (74/114) | 90.0 (297/333) |
| P-value | 1.00 | 1.00 | - | - | - |
5. Discussion
As a critical tool for the preoperative evaluation of endometrial cancer, MRI is widely utilized in diagnosing cervical stromal invasion due to its non-invasive nature, high tissue contrast, and multi-sequence and multi-parameter imaging capabilities (15). This study demonstrates that cervical enhancement on DCE-MRI images presents varied characteristics. Consequently, not all patients require delayed phase imaging, as the arterial phase of DCE-MRI can achieve comparable diagnostic efficacy. However, when the cancer is located in the lower uterine segment or at the internal os of the cervix, and there is a clustered distribution of Nabothian cysts in the cervical mucosa, determining the presence of cervical stromal invasion in endometrial cancer becomes challenging.
Not all patients require delayed-phase scanning due to the variation in cervical enhancement across different stages of DCE-MRI imaging. The cervix is a complex, heterogeneous tissue composed of collagen and elastic fibers that respond to changes in the pelvic environment (16, 17). Pelvic blood vessels have extensive collateral circulation, with the pelvic arterial system forming connections between the aorta, internal iliac artery, external iliac artery, and femoral artery (18, 19). Anatomically, the uterus receives its primary blood supply from the uterine artery, with cervical blood flow being comparatively lower than that of the uterine body (20). The uterine artery is a branch of the internal iliac artery (21), and variations in physiological state and environmental conditions can alter uterine artery blood flow (22, 23). The uterine artery and the pudendal artery are extensively anastomosed to supply the cervix. When blood flow in the uterine artery is affected, the anastomotic vascular network ensures continued blood supply to the cervix (19, 24). Variations in cervical blood flow result in differing degrees of cervical enhancement. Thus, radiologists can develop an optimal scanning plan for patients with suspected cervical stromal invasion in endometrial cancer by evaluating the unique cervical enhancement patterns in DCE-MRI.
Accurate diagnosis of cervical stromal invasion is essential for determining the appropriate treatment strategy (25). Literature reports indicate that MRI sensitivity for detecting cervical stromal invasion ranges from 22% to 100%, with specificity between 86% and 100%. For DCE-MRI, sensitivity in diagnosing cervical stromal invasion ranges from 58% to 91%, and specificity from 78% to 97%. Our findings demonstrated that the sensitivity of DCE-MRI in identifying cervical stromal invasion was 66.4% in the arterial phase and 69.1% in the delayed phase, with specificities of 87.9% and 88.2%, respectively, aligning with previously reported diagnostic efficacy ranges. Currently, no studies compare the diagnostic efficacy of DCE-MRI in the arterial versus delayed phases (13, 26-28). Our analysis revealed no statistical difference between the two diagnostic phases. Consequently, this study provides valuable data supporting the feasibility of using the arterial phase of DCE-MRI for assessing cervical stromal invasion in endometrial cancer, suggesting that the delayed phase may not be necessary for this evaluation.
Literature reports indicate that cervical mucosa enhancement occurs earlier than stromal enhancement following contrast agent injection (12). This study found that cervical mucosal enhancement appeared similarly in both the arterial and delayed phases with DCE-MRI. Cervical stromal invasion in endometrial cancer was observed as a relatively low-signal area during both phases of DCE-MRI. In this context, the cluster-like distribution of non-enhanced cervical cysts can obscure the boundary between cervical mucosa and stroma on MRI images, affecting diagnostic accuracy. These findings align with Lin et al. (26), who highlighted that factors influencing the assessment of cervical stromal invasion in endometrial cancer include the location of the lesion, particularly in the lower uterine segment or isthmus. When cancer is located in the lower segment of the uterus or internal os of the cervix, diagnostic limitations arise in both the arterial and delayed phases of DCE-MRI. In such cases, the clustered distribution of cervical cysts further complicates the diagnosis, making it challenging for MRI to accurately determine cervical stromal involvement.
There are some limitations in this study. The sagittal image was primarily used for evaluating cervical stromal invasion, which may result in some diagnostic deviation. Therefore, axial and coronal images were also observed to minimize this impact. As a retrospective study, patients with endometrial cancer prior to 2023 were included to avoid the influence of the new 2023 endometrial cancer staging guidelines on the results. For future research, we aim to explore potential differences in cervical enhancement and the diagnosis of cervical stromal invasion by DCE-MRI between premenopausal and postmenopausal patients.
In conclusion, accurate disease staging and appropriate treatment planning at diagnosis are critical to the successful management of patients with endometrial cancer. Our results demonstrate that the arterial phase of DCE-MRI provides valuable information for diagnosing cervical stromal invasion. Given the increasing incidence of endometrial cancer, developing an efficient scanning protocol can significantly save both time and cost.