1. Background
Acute basilar artery occlusion (BAO) is a type of ischemic cerebrovascular disease that, while not highly prevalent, has a high mortality and disability rate. When treated solely with medical therapy, the poor outcome rate for BAO exceeds 80% (1-3), and can be even higher. With the advancement of neurointerventional devices, new drugs, techniques, and concepts, endovascular treatment (EVT) has significantly improved the prognosis of BAO patients. However, the outcome for BAO patients remains worse than that for patients with acute large vessel occlusion in the anterior circulation, and the treatment process consumes considerable economic and medical resources (4).
Recent studies have shown that the clinical outcome of BAO is influenced by multiple factors, with the most attention given to collateral circulation and thrombus burden (5). The basilar artery apex refers to the top of the basilar artery in the human brain, resembling a "Y" shape, where five blood vessels converge. These vessels include the basilar artery, superior cerebellar artery, and bilateral posterior cerebral arteries. Due to the anatomical characteristics of the posterior circulation, collateral circulation is significantly less developed than in the anterior circulation. The apex of the basilar artery is situated at the brain's base, where it divides into the posterior cerebral arteries. This region is anatomically intricate and may pose difficulties for clear imaging. Comprehensive imaging is essential for the precise evaluation of the vessel's architecture and any possible irregularities.
The most critical aspect of collateral compensation in the posterior circulation is the opening of the posterior communicating artery, which relies on the Circle of Willis to supply blood to the posterior circulation, thereby improving the prognosis of BAO patients undergoing EVT. This anatomical basis explains the impact of the basilar artery apex on prognosis. The basilar artery is essential for delivering blood to the brainstem and posterior cerebral hemispheres. Thrombosis or stenosis of the basilar artery can result in severe ischemic incidents, including strokes. Advanced imaging modalities are essential for assessing the degree of obstruction and formulating suitable treatment strategies. The apex of the basilar artery is a crucial region with a significant risk for severe vascular disorders. Advanced imaging techniques are frequently required for precise diagnosis, treatment guidance, and outcome monitoring.
2. Objectives
This study specifically assessed the influence of basilar artery apex imaging on the prognosis of BAO. By distinguishing lesion patterns between 90-day modified Rankin Scale (mRS) scores, our findings could enhance the clinical understanding of BAO and inform prognosis, patient management, and potential therapeutic strategies.
3. Patients and Methods
3.1. Study Population
A retrospective cohort study was conducted on the clinical data of patients with BAO who received EVT at Jiangyin People's Hospital (Advanced Stroke Center of China) from January 2019 to December 2023. All eligible patients within the study period who met the inclusion criteria were included; no a priori sample size calculation was performed due to the retrospective nature of the study.
Inclusion criteria were as follows: (1) Age > 18 years; (2) completed computed tomography angiography (CTA) before treatment; (3) confirmed as BAO by digital subtraction angiography (DSA); (4) received either intra-arterial stent retrieval or intravenous thrombolysis-bridged endovascular stent retrieval within 24 hours of onset; (5) distal blood flow reached modified Treatment in Cerebral Ischaemia Scale (mTICI) grade 2b or 3 after EVT.
Exclusion criteria included: (1) Poor imaging quality; (2) pre-onset mRS score ≥ 1; (3) previous thrombectomy treatment; (4) lack of 90-day mRS and unavailable CT image and clinical data.
All patients were followed up for 90 days post-treatment to assess outcomes.
3.2. Endovascular Treatment
All patients underwent EVT. The procedure involved puncturing the femoral artery using the modified Seldinger technique, inserting an 8F arterial sheath, and confirming BAO by digital subtraction angiography (DSA). After positioning the long sheath or guide catheter, the intermediate catheter was advanced to the occlusion site. Initial aspiration was followed by microcatheter guidance over a microwire past the occlusion. Upon confirming true lumen placement, the stent was deployed, left for 3 - 5 minutes, and then retracted to remove the thrombus. Multiple passes were made if necessary. Severe stenosis or vascular dissection warranted stent placement. Post-operatively, tirofiban was administered via continuous infusion, switching to dual antiplatelet therapy after a 24-hour CT confirmed no hemorrhage. To minimize observer bias, radiological assessments were independently conducted by neuroradiologists blinded to patient outcomes. Recall bias in outcome collection was reduced through standardized follow-up protocols.
3.3. Patient Clinical and Radiological Variables
Data were collected on basilar artery apex imaging (CTA + DSA), 90-day mRS score, age, gender, pc-CTA score, blood glucose levels, comorbidities, platelet count, trial of Org 10172 in acute stroke treatment (TOAST) classification, time to hospital, onset-to-puncture duration, and puncture-to-recanalization interval for patients diagnosed with BAO using DSA and CTA. The basilar artery apex was defined anatomically as the bifurcation of the basilar artery into the posterior cerebral arteries (PCAs), typically accompanied by the superior cerebellar arteries (SCAs), located anterior to the pontomesencephalic junction with an approximate diameter of 2 mm. The "available image of the basilar artery apex" was determined when this bifurcation was clearly and continuously visualized on CTA or DSA. In contrast, images lacking this depiction due to thrombus obstruction, poor contrast filling, or suboptimal quality were classified as "unavailable". Neuroradiologists independently assessed these imaging features to determine image availability status.
The primary outcome was a favorable functional outcome at 90 days (mRS ≤ 2). Exposure variables included basilar artery apex morphology and other clinical characteristics. Potential confounders considered were age, comorbidities, and time-to-treatment intervals. The imaging results were assessed independently by two to three neuroradiologists who were blinded to the patients' clinical outcomes. Endovascular treatment was performed by four professors from our center, using three DSA devices from Philips Company. While different operators performed the interventions, all operators used similar techniques to minimize variability.
3.4. Patient Outcome
Patients were categorized into a favorable outcome group (mRS ≤ 2) and an unfavorable outcome group (mRS > 3) according to the 90-day mRS score. The 90-day mRS was collected by telephone interview or outpatient visit 90 days after onset.
3.5. Statistical Analysis
Continuous variables are presented as the median and interquartile range (IQR). Categorical data are presented as frequency and percentage and tested using Pearson's chi-square test or Fisher's exact test. All tests are two-sided, and a P-value < 0.05 is considered statistically significant. For screening influencing factors, variables between the two groups are compared using least absolute shrinkage and selection operator (LASSO) regression. The LASSO regression was used to reduce dimensionality and select variables, with lambda values chosen through cross-validation. Finally, univariate and multivariate logistic analyses are used to determine predictive factors. Predictive factors were identified through univariate and multivariate logistic regression. A receiver operating characteristic (ROC) curve is used to evaluate the predictive ability of the predictive factors. The ROC analysis evaluated model discrimination, and calibration curves (1,000 bootstrap resamples) assessed model fit. A calibration plot was generated with 1,000 bootstrap resampling to depict the correlation between the actual favorable outcome and the predicted probability of a favorable outcome. Logistic analyses and ROC curves were executed using SPSS 20 (IBM Corp. Released 2011. IBM SPSS statistics for Windows, version 20.0. Armonk, NY: IBM Corp.), while variable selection in LASSO regression was carried out using R language (4.4.0). The prognostic capability of basilar artery apex imaging for BAO was evaluated using ROC curves.
4. Results
4.1. Baseline Data and Clinical Information
Of the 62 patients with BAO who were admitted to our institution and underwent EVT, 3 patients were not included in the study population. Our study included 59 patients who fulfilled the inclusion criteria, with 19 (32.2%) in the favorable outcome group and 40 (67.8%) in the unfavorable outcome group. No substantial differences were observed between the two groups regarding gender, pc-CTA, time to hospital, onset-to-puncture time, puncture-to-recanalization time, triglycerides (TG), platelet count (Plt), TOAST classification, and comorbidities (alcohol consumption, hypertension, diabetes, atrial fibrillation) (P > 0.05).
The therapeutic relevance of variations in age, blood glucose (Glu), and LDL levels between favorable and unfavorable outcome groups might yield critical insights into the determinants affecting patient prognosis, especially in illnesses like stroke, cardiovascular diseases, or other vascular disorders. Statistically significant differences (P < 0.05) were observed in age, blood Glu, LDL, presence of basilar artery apex, and admission National Institutes of Health Stroke Scale (NIHSS) score, as illustrated in Table 1.
| General information | Good outcome group (n = 19) | Poor outcome group (n = 40) | χ2/t/Z | P-value |
|---|---|---|---|---|
| Gender (male/female) | 16/3 | 31/9 | 24.6 | 0.394 |
| Age (y) | 52.6 ± 11.75 | 61.18 ± 10.51 | 2.21 | 0.033 b |
| Pc-CTA | 3.90 ± 1.45 | 4.47 ± 1.61 | 1.29 | 0.2 |
| Glu (mmol/L) | 6.95 ± 2.25 | 9.78 ± 3.15 | 2.231 | 0.031 b |
| LDL (mmol/L) | 2.75 ± 0.43 | 2.28 ± 0.66 | -2.091 | 0.043 b |
| TG (mmol/L) | 1.31 [0.87,2.05] | 1.3 [0.86,2.17] | 0.12 | 0.905 |
| Plt (mmol/L) | 212.5 [162.5, 244.25] | 181 [144.25, 220.5] | -1.127 | 0.266 |
| Time to Hospital (min) | 170 [90, 495] | 180 [120, 300] | -0.437 | 0.67 |
| Onset-to-puncture time (min) | 60 [30, 92.5] | 50 [40, 60] | -0.828 | 0.428 |
| Puncture-to-recanalization time (min) | 105 [48.75,142.5] | 120 [60, 180] | 1.317 | 0.195 |
| The available image of basilar artery apex | 14 (73.68) | 6 (15) | 19.528 | < 0.001 c |
| TOAST classification | 0.518 | 0.764 | ||
| Atherosclerosis | 10 (52.63) | 23 (57.5) | ||
| Cardioembolism | 5 (26.31) | 11 (27.5) | ||
| Others, SOE or SUE | 4 (21.05) | 6 (15) | ||
| Admission NIHSS score | 14 (12, 18) | 26 (16, 37) | -2.019 | 0.036 b |
| Basic diseases | ||||
| Smoking | 1 (5.2) | 2(5) | 1.896 | 0.271 |
| Alcohol consumption | 6 (31.57) | 19(47.5) | 0.27 | 0.87 |
| Hypertension | 9 (56.25) | 22 (55) | 0.096 | > 0.99 |
| Diabetes | 0 (0) | 7 (17.5) | 1.659 | 0.573 |
| Atrial fibrillation | 4 (21.05) | 6 (15) | 2.043 | 0.31 |
Baseline Data and Clinical Information a
4.2. Least Absolute Shrinkage and Selection Operator Regression Screening Results
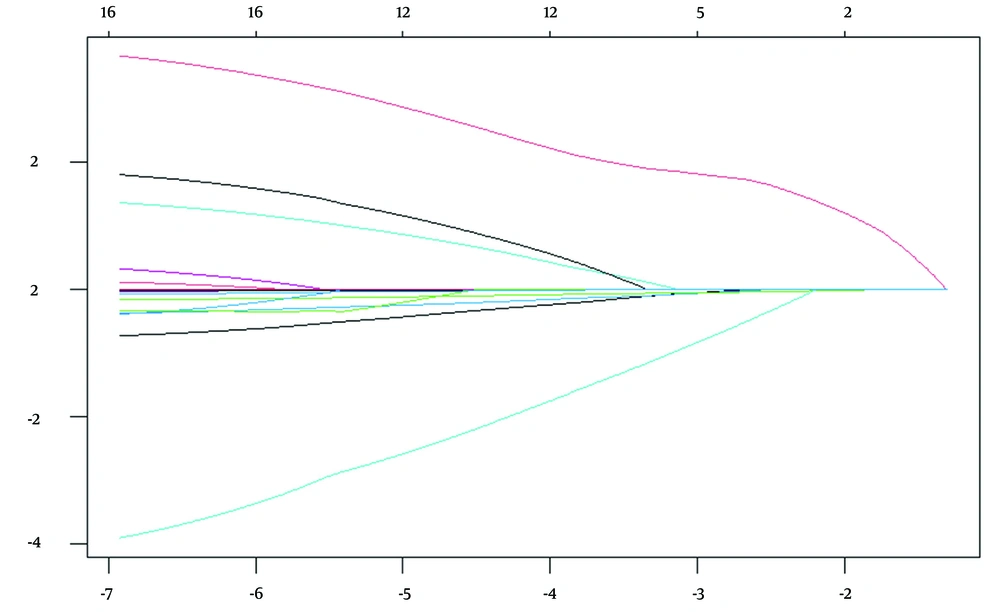
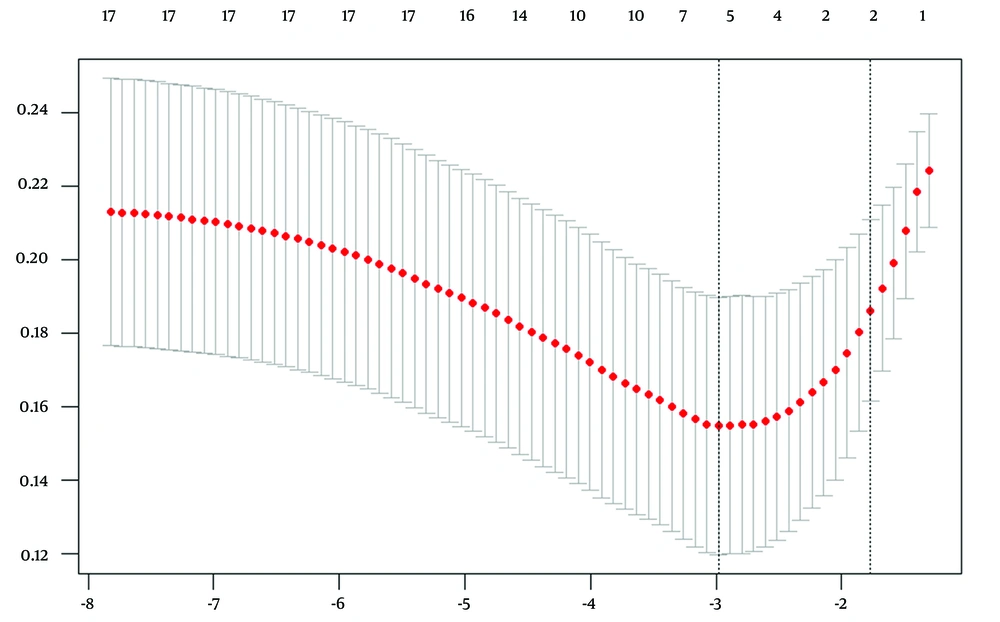
Least absolute shrinkage and selection operator regression analysis was used to evaluate 14 potential influencing factors, utilizing the 90-day mRS score (≤ 2 / > 3) as the outcome variable. As the penalty coefficient λ varied, the coefficients of the influencing factors were progressively diminished. Cross-validation with Lambda (0.05071808) identified the most significant variables, pc-CTA, the accessible image of the basilar artery apex, admission NIHSS score, smoking, and Glu. Cross-validation utilizing Lambda.1se (0.1699869) identified the minimal set of variables, the accessible image of the basilar artery apex and the admission NIHSS score, as shown in Figures 1 and 2.
The upper horizontal axis represents the number of selected variables; the lower horizontal axis represents Log (λ); the vertical axis represents model error (binomial deviation). The two dashed lines from left to right indicate Lambda.min and Lambda.1se, respectively. Lambda.min corresponds to the model with the lowest error, while Lambda.1se corresponds to the simplest model that still performs within one standard error of the best model. The red point indicates the chosen λ value, which is either Lambda.min or Lambda.1se for building the model.
4.3. Univariate and Multivariate Analyses
In the univariate logistic analysis, pc-CTA (P = 0.007), the available image of the basilar artery apex (P < 0.001), admission NIHSS score (P = 0.003), and Glu (P = 0.035) were found to be significantly associated with a good outcome. The multivariate logistic regression analysis identified the available image of the basilar artery apex (OR, 8.868; 95% CI, 1.683 - 36.727) and admission NIHSS score (OR, 0.917; 95% CI, 0.854 - 0.984) as prognostic factors of a good outcome at 90 days (Table 2).
| Variables | Univariate logistic analysis | Multivariate logistic regression | ||
|---|---|---|---|---|
| OR (95%CI) | P-value | OR (95%CI) | P-value | |
| Available image of basilar artery apex | 0.067 (0.017,0.241) | < 0.001 | 8.868 (1.683,36.727) | 0.01 |
| Admission NIHSS Score | 0.91 (0.856,0.968) | 0.003 | 0.917 (0.854,0.9841) | 0.017 |
| Smoking | 3.924 (0.988,15.721) | 0.052 | - | - |
| Glu | 0.75 (0.627,0.983) | 0.035 | 0.822 (0.611,1.107) | 0.092 |
| Pc-cta | 0.557 (0.364,0.851) | 0.007 | 0.807 (0.470,1.385) | 0.436 |
Univariate and Multivariate Analyses
4.4. Receiver Operating Characteristic Curve Construction
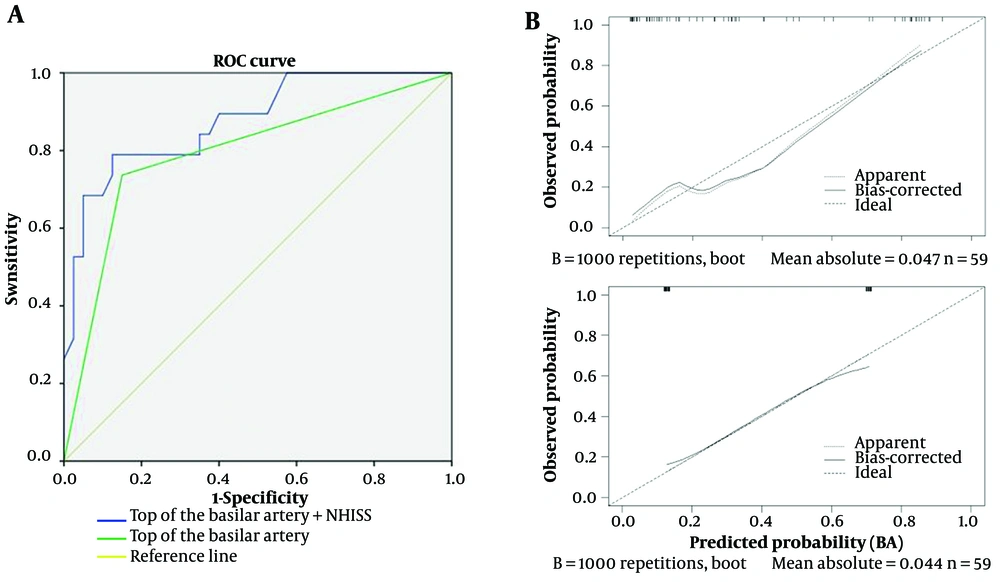
The ROC curve plotted for predicting prognosis based on the available image of the basilar artery apex shows an area under the curve (AUC) of 0.793 (95% CI, 0.66 - 0.927) with 73.7% sensitivity and 85% specificity. Furthermore, the AUC combining the available image of the basilar artery apex and admission NIHSS score is 0.877 (95% CI, 0.781 - 0.913) with 79.8% sensitivity and 90.7% specificity, as shown in Figure 3A. In Figure 3B, the points of the calibration plot for the probability of a favorable outcome for patients with BAO are close to the 45° line, suggesting a positive correlation between predictions by the nomogram and actual observations (Figure 3). The mean squared error of the prognostic model was 0.047 and 0.044, also indicating a strong level of calibration performance of the model we built.
A, Receiver operating characteristic (ROC) curve for predicting prognosis using the available image of the basilar artery apex and Admission National Institutes of Health Stroke Scale (NIHSS) Score. The curve shows the area under the curve (AUC) values for the models with the available image alone and the combination with NIHSS. B, Calibration plot comparing the predicted probability of a favorable outcome for basilar artery occlusion (BAO) patients with actual observations. The points are close to the 45° line, indicating a positive correlation between the nomogram predictions and actual outcomes.
4.5. Case Examples
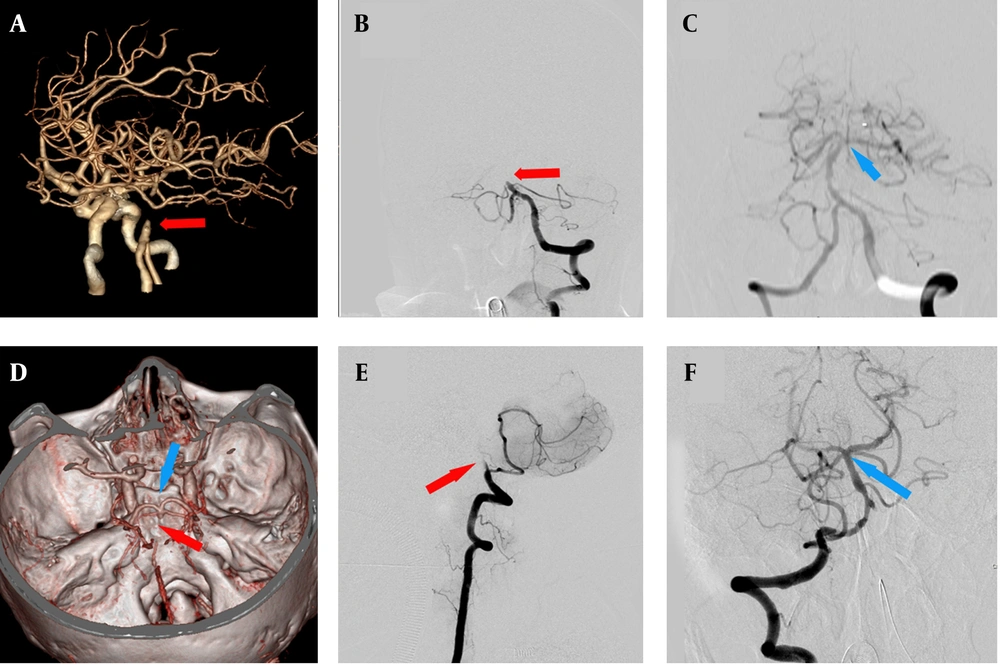
Figure 4 illustrates the changes of the basilar artery apex during EVT for BAO. Among all 59 patients treated with EVT, a good outcome was achieved in 19 patients (32.2%), and the available image of the basilar artery apex was obtained in 14 patients (73.68%).
Case examples of endovascular treatment (EVT) for basilar artery occlusion (BAO) and changes of basilar artery apex. A patient with BAO initially presented with no visible image of the basilar artery apex and basilar artery interruption, as indicated by the red arrow on computed tomographic angiography (CTA, A) and digital subtraction angiography (DSA, B). After EVT, the basilar artery apex image became visible, as indicated by the blue arrow in C. Another patient with BAO presented with an initially visible basilar artery apex and basilar artery interruption, as indicated by the blue and red arrows on CTA (D) and DSA (F). Following EVT, the basilar artery apex remained visible, as indicated by the blue arrow in E.
5. Discussion
In our study, we identified a simple indicator (available image of the basilar artery apex) for assessing the favorable outcome of patients with BAO. We found that admission NIHSS, blood glucose, pc-CT, and smoking are potential predictors of favorable outcomes for BAO patients treated with EVT. By using LASSO/logistic regression to minimize confounding factors as much as possible, we concluded that basilar artery opacification has good performance (AUC, 0.799; 95% CI, 0.66-0.927) and calibration ability (mean squared error = 0.044) for favorable outcomes after EVT. In sensitivity analyses, when combined with admission NIHSS, the predictive value is further enhanced (AUC 0.877; 95% CI, 0.781-0.913; mean squared error = 0.047). This indicates that the association between the available image of the basilar artery apex and favorable outcome remained robust. These results suggest that our primary findings are unlikely to be substantially influenced by potential biases or methodological choices.
In the present study, endovascular mechanical thrombectomy techniques have made significant advances in the treatment of acute ischemic stroke. Current research indicates that selected anterior circulation large vessel occlusive acute ischemic strokes benefit significantly from EVT (6). Additionally, the results of the Basilar Artery International Cooperation Study (BASILAR) demonstrate the safety and efficacy of EVT for acute BAO (7). Studies now recognize that favorable outcomes are associated with treatment timing, successful recanalization, NIHSS score on admission, stroke etiology, and collateral circulation (8, 9).
Currently, the prognosis prediction of clinical outcomes primarily relies on imaging assessments, such as pc-CS, pc-CTA, and BATMAN scoring systems for BAO (10-12). These classifications score the main vessels, branches, and compensatory mechanisms of the posterior circulation. Among them, pc-CS and BATMAN scores indicate better compensation with higher scores, while pc-CTA scores indicate poorer compensation with higher scores. Recently, Gao et al. (13) reported a new collateral circulation grading system (ACGS-BAO), which consists of four levels with higher grades indicating better compensation. Pc-CTA scoring primarily assesses thrombus burden in BAO, BATMAN scoring evaluates thrombus location and collateral circulation in the basilar artery. ACGS-BAO grading and pc-CS primarily target posterior circulation after BAO. These four scoring systems have certain limitations and are cumbersome in clinical applications, especially when patients score high in pc-CS and pc-CTA but low in BATMAN scoring (14). Among these systems, BATMAN scoring is more accurate. ACGS-BAO grading has not yet shown positive results in influencing prognosis. However, all these scoring systems underscore the importance of collateral circulation in the prognosis of BAO.
Due to the high mortality and disability rate associated with outcomes from BAO, patients with a 90-day mRS score of 3 are typically assigned to the good outcome group in most studies. However, to confirm the value of the available image of the basilar artery apex, we assigned them to the poor outcome group. In this study's LASSO regression analysis, cross-validation identified five key factors corresponding to Lambda min, including pc-CTA, the available image of the basilar artery apex, admission NIHSS score, smoking, and Glu. These factors are critically associated with the onset and prognosis of BAO. As the λ value increased, the available image of the basilar artery apex and admission NIHSS score emerged as the only two statistically significant variables in the model, which was subsequently validated in the ROC curve.
The basilar artery apex is the junction consisting of five branch vessels, located at the front of the junction between the pons and midbrain, with a diameter of approximately 2 mm. During our study, our neuroradiologists clarified the basilar artery apex by confirming the junction of the basilar artery, posterior cerebral artery, and superior cerebellar artery, thereby determining the available imaging for the basilar artery apex. In our study, the fetal-type posterior cerebral artery was quite common, mainly unilateral, and thus had a minimal impact on our assessment of the basilar artery apex. However, the presence of bilateral fetal-type posterior cerebral arteries often caused confusion in confirming the imaging of the basilar artery apex. Yet, such patients typically exhibited mild symptoms and rarely underwent EVT. Moreover, a clearer diagnosis through DSA imaging could reduce the confusion that such cases might introduce to our study.
The ability of basilar artery apex imaging and admission NIHSS score to predict prognosis post-thrombectomy in BAO analysis may be related to collateral circulation opening. In our study, the "available image of the basilar artery apex" was precisely defined as a clearly visualized anatomical junction of the basilar artery with the posterior cerebral arteries and superior cerebellar arteries, seen on either CTA or DSA. It is anatomically located at the anterior portion of the pons-midbrain junction, typically measuring approximately 2 mm in diameter. We considered the image as "available" only when this region was well visualized without motion artifacts, poor contrast, or thrombus obscuration. The visibility of this region may suggest lower clot burden, better perfusion, and preserved collateral pathways — factors that contribute to a more favorable prognosis. These characteristics, as shown in our multivariate and ROC analyses, indicate that the imaging clarity of the basilar artery apex is not merely a structural observation but a clinically meaningful marker linked to improved 90-day mRS scores.
The basilar artery apex configuration and its connection to the Circle of Willis could impact prognosis. However, our study does not clarify whether the "available image" has any anatomical or clinical correlation with these factors. The visibility of the basilar artery apex on imaging modalities such as CTA or DSA may serve as a surrogate marker for the integrity of collateral circulation. Anatomically, the basilar artery apex is a crucial junction where perforating arteries that supply vital regions, including the thalamus and midbrain, emerge. These regions are pivotal in maintaining cerebral function and may directly influence the outcome following endovascular therapy. Further investigation into the anatomical variations at this junction and their relationship with clinical outcomes could provide deeper insights into the mechanisms underlying the observed correlation. Addressing this relationship would strengthen the interpretability of our findings and provide a more robust scientific framework for our conclusions.
The ability of basilar artery apex imaging and admission NIHSS score to predict prognosis post-thrombectomy in BAO analysis may be related to collateral circulation opening. Following BAO, collateral circulation primarily involves the posterior communicating artery, anastomoses of the meningeal branches from the anterior circulation, and cerebellar arteries, predominantly through the posterior communicating artery. The basilar artery apex serves as a crucial transit point for these collateral circulations. Additionally, perforating arteries at the top of the basilar artery supply vital regions such as the thalamus and midbrain, providing valuable time for endovascular intervention and enhancing thrombectomy outcomes.
Cerebral stenting, performed acutely during endovascular therapy for persistent stenosis due to cerebral atherosclerotic disease and subacutely for severe, unresponsive stenosis, is becoming more accepted. Luo et al. (15) observed 97 patients with severe symptomatic cerebral vertebrobasilar stenosis (70 - 99%), 30 had BA, 55 had V4, and 12 had V4-BA. Their group had 96 successful stent placements. Two fatalities, two brain hemorrhages, and seven ischemic events occurred. The Mori classification of intracranial artery stenosis was used to classify lesions as type A (less than 5 mm, concentric or moderately eccentric, not completely occlusive), type B (tubular lesions 5 - 10 mm, extremely eccentric or fully occluded), and type C. Mori C lesions were treated with self-expanding stents, while Mori A lesions using Apollo stents. Complications were more common in Mori C lesions compared to Mori A (P = 0.008) and Mori B (P = 0.047).
Another multicenter prospective registry by Gao et al. (13) examined the safety and efficacy of stenting within 30 days for patients with 70 - 99% severe symptomatic stenosis. Of 159 balloon-mounted stent patients, 48 had basilar and intracranial vertebral artery stenosis. Of 141 patients who underwent balloon angioplasty and self-expanding stents, 46 had basilar artery stenosis and 25 had intracranial vertebral stenosis. The cumulative 30-day stroke, transient ischemic attack (TIA), and death rate was 4.3%. Revascularization was successful in 97.3%.
Acute stenting works for BAO thrombus retrieval failures. In 93 Chinese patients, rescue stenting following EVT failure was tested for safety and efficacy in BAO. Strokes were 76.2% atherosclerotic, 7.1% cardioembolic, and 16.7% other or unknown. TICI 2b/3 was achieved by 75 rescue stenting patients (92.6%). Additionally, rescue stenting improved 90-day mRS 0-3 outcomes (51.9% vs. 16.7%; P = 0.023) without increasing spontaneous intracerebral hemorrhage (sICH) incidence. Rescue stenting decreased mortality (18.5% vs. 58.3%; P = 0.006). The study has limitations such as observational technique, small sample size in the non-stented cohort, only including patients with NIHSS ≥ 10, and geographical variations in intracranial atherosclerotic disease (ICAD) prevalence in China (15).
Futile recanalization in posterior circulation during EVT is prevalent in multicenter studies (16). This study's ROC curve findings show a high probability for the available image of the basilar artery apex and admission NIHSS score in predicting prognosis, offering clinical assessment support to reduce futile recanalization before treatment initiation. The multivariate logistic regression analysis (Table 2) indicated that the presence of a picture of the basilar artery apex (OR, 8.868; 95% CI, 1.683 - 36.727) could predict favorable outcomes following EVT for VBAO. This study reveals that the visibility of the basilar artery apex in CTA/DSA is directly linked to a favorable prognosis. Nevertheless, owing to the limited sample size and single institution, additional comparisons with other variables are imperative. Nonetheless, these streamlined image interpretations provide operational benefits in clinical environments.
This retrospective study may exhibit bias due to its limited sample size, highlighting the need for larger prospective investigations to validate its applicability. Recent randomized trials have had inconclusive outcomes, leaving lingering doubts. Future research employing standardized clinical evaluations and imaging methods is essential to enhance our comprehension of the efficacy, safety, selection criteria, and optimal technical methodologies for endovascular therapy in patients with BAO. Currently, a judicious strategy involves selecting patients exhibiting more severe stroke symptoms with reduced infarct loads. Clinical parameters related to posterior circulation stroke severity and outcomes may provide clarity, whereas enhanced imaging during the hyperacute period may offer numerous advantages for decision-making. Despite existing information gaps, the majority of professionals in our area concur that a reperfused basilar artery is preferable to an occluded one for patients with viable brain tissue to preserve (17).
In conclusion, this study indicates that the available image of the basilar artery apex confirmed by CTA/DSA within 24 hours of onset in acute BAO patients predicts better clinical outcomes post- EVT, providing a convenient method for predicting clinical prognosis.