1. Background
Breast cancer is one of the most prevalent cancers in women. The Breast Imaging Reporting and Data System (BIRADS) is used for the radiologic classification of breast lesions and is a highly accurate method for predicting the risk of malignancy. The BIRADS categorization is performed by combining ultrasound, mammography, and magnetic resonance imaging (MRI) findings. The most commonly used histopathologic sampling method for lesions suspicious for malignancy (BIRADS 4 and 5) is imaging-guided core needle biopsy (CNB). The CNB has replaced excisional biopsy (EB) in the sampling of lesions detected by imaging and is now considered the first-choice method in diagnosis because it shows equivalent accuracy to EB (1). Pathologic results are considered concordant if they provide an acceptable explanation for the imaging features and discordant if they do not. Parikh and Tickman (2) described five possible outcomes of imaging-pathology correlation. One of them, ‘discordant benign lesion’, is suspicious for malignancy at imaging. Wan et al. (3) defined this as false discordance in their study. When CNB findings are incompatible with clinical or radiologic findings, additional surgery is required to exclude a possible malignancy. There are not many studies in the literature addressing the rate of upgrade to malignancy with the excision of lesions that are found to be benign by CNB, and the reported percentages vary widely (0% - 53.8%) (4).
2. Objectives
In this study, we compared the pathology results of patients with radiologically BIRADS category 4 and 5 lesions who underwent excision, with no histopathological malignancy detected by CNB. Our aims in this study were to determine the frequency of discordant benign lesions, evaluate the rates of upgrade to malignancy in excised patients, and examine benign conditions that may be confused with malignancy radiologically.
3. Patients and Methods
3.1. Patient Selection
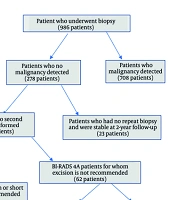
In this study, 986 patients who underwent CNB between 2020 and 2023 in our hospital were retrospectively analyzed. Histopathologically, malignancy was detected in 708 patients at the first biopsy and 26 patients at the repeat biopsy. Among the patients with no evidence of malignancy in both biopsies, 73 female patients who underwent EB/lumpectomy due to persistent clinical-radiologic-pathologic suspicion or social indications were included in the study. The remaining 233 patients were stable at a 2-year imaging follow-up and were considered benign. Breastfeeding patients and pregnant women were not included in the study. A summary of patient selection is shown in Figure 1. The study was approved by the local ethics committee of our hospital (Decision No: 2023/68).
Clinical-radiological-pathological discordance is the situation when malignancy is suspected due to imaging methods, clinical findings, or family history, but pathological malignancy cannot be completely excluded by CNB. In these cases, excision of the entire lesion is recommended with the decision of a multidisciplinary committee.
3.2. Imaging Technique and Biopsy Procedure
Radiologic evaluation of imaging and ultrasound-guided biopsy were performed by two radiologists with 8 and 30 years of experience in breast radiology. Microlobulated and irregular contours on ultrasound, presence of microcalcifications on mammography, contrast enhancement pattern with type 2-3 time-signal curve on dynamic breast MRI, and diffusion restriction on diffusion-weighted images are findings associated with malignancy and used in BIRADS categorization (5). Final BIRADS categorization was decided by ultrasound in 5 patients, mammography in 19 patients, and MRI in 49 patients. Age, breast composition, tumor maximum size, and suspicious findings for malignancy were recorded with imaging methods.
A Samsung trademark ultrasound device and a 2.0 - 14.0 MHz frequency LA2-14A linear probe were used. Standard craniocaudal and mediolateral oblique radiographs were obtained in all patients (Mammomat Revelation, Siemens, Erlangen, Germany). Magnetic resonance imaging was performed with a 1.5 Tesla MRI (Magnetom Aera, Siemens, Erlangen, Germany) using a 4-channel breast coil. Fat-suppressed T1-weighted (T1W) pre-contrast images [repetition time (TR): 476 ms, echo time (TE): 11 ms] and T2-weighted (T2W) images (TR: 2250 ms, TE: 56 ms) were obtained in all MRI scans. Axial diffusion-weighted images were also obtained at b-values of 0, 500, and 1000 s/mm2. Apparent diffusion coefficient maps were depicted automatically using software provided by the manufacturer (Syngo.via, Siemens, Erlangen, Germany). After intravenous injection of a gadolinium contrast agent, 5 dynamic post-contrast fat-suppressed T1W images (TR: 4.53 ms, TE: 1.82 ms) were obtained. Dynamic curves of percent enhancement versus time were obtained for lesions at small regions of interest, positioned on the brightest portion of the lesion.
After cleaning the area appropriate for biopsy under ultrasound guidance, 2% lidocaine was injected. Then, at least three specimens were taken from different parts of the lesion using an 18-gauge, 15 cm fully automatic biopsy gun through a 17-gauge coaxial needle. The specimens were fixed in a 10% formalin solution and standardized for histologic evaluation. Both CNB and EB specimens were retrospectively evaluated by the same pathologist with 25 years of experience. The pathologist was blind to clinical and radiological data. The EB results were classified as benign, borderline, and malignant.
3.3. Statistical Analysis
Analysis of the data was performed using SPSS (IBM Corp. Released 2019. IBM SPSS Statistics for Windows, Version 26.0. Armonk, NY: IBM Corp). The normality distribution of continuous variables was evaluated by the Shapiro-Wilk test. Independent group comparisons were performed with the independent samples t-test for variables with a 'Normal Distribution'; mean and standard deviation values were presented. Independent group comparisons of variables that did not show normal distribution were performed with the Mann-Whitney U test; median and interquartile range (IQR: 25% - 75%) values were given. Categorical independent variables were presented as frequencies and percentages in cross tabulations. The Pearson chi-square test was performed for the difference of distribution in independent groups if the minimum expected value was above 25, and Yates correction was used if it was in the range of 5 - 25. In cases where the expected value was below 5 and above 1, Fisher’s exact test was used. Univariate odds ratios were calculated, and 95% confidence intervals (CI) were determined according to the Mantel-Haenszel method, and significance was analyzed. Variables with significant differences were included in the multivariate logistic regression analysis as independent variables, and multivariate odds ratios were calculated by the backward stepwise (Wald) method. Type-1 error α: 0.05 was chosen, and all tests were performed as two-tailed (right α/2 = 0.025 and left α/2 = 0.025). The difference between the groups was considered statistically significant if the P-value was < 0.05.
4. Results
In our study, the discordance rate after excision and 2-year follow-up was 1.9% (19/986). Of the 73 lesions with EB, 53 were benign, 1 was borderline, and 19 were malignant lesions. The distribution of histologic subtypes according to excision results is shown in Table 1.
| Histological sub-type | Number of patients (n) |
|---|---|
| Malignant | 19 |
| Invasive ductal carcinoma | 4 |
| Encapsuled papillary carcinoma | 2 |
| Microinvasive ductal carcinoma in situ | 1 |
| Mucinous carcinoma | 1 |
| Ductal carcinoma in situ | 11 |
| Borderline phylloid tumor | 1 |
| Benign | 53 |
| Fibroadenoma | 21 |
| Intraductal papilloma | 9 |
| Atypical ductal hyperplasia | 4 |
| Apocrine metaplasia | 3 |
| Myofibroblastic benign stromal tumor | 3 |
| Tubular adenoma | 2 |
| Fibroepithelial changes | 2 |
| Adenosis | 2 |
| Columnar cell hyperplasia | 2 |
| Radial scar | 2 |
| Inflammatory granulomatous changes | 2 |
| Fat necrosis | 1 |
The median tumor size was calculated as 20 mm in the malignant group and 24 mm in the benign-borderline group, with no statistically significant difference between them (P = 0.646). The mean patient age was 50.2 ± 9 years in the malignant group and 42.1 ± 12.2 years in the benign-borderline group, and it was significantly lower in the benign group (P = 0.011).
When the patients were divided according to breast fibroglandular tissue density, 21 patients had type A (0 - 25%, almost completely fatty) and type B (25 - 50%), while 52 patients had type C (50 - 75%) and type D (75 - 100%, sclerosed). There was no significant difference in breast composition between the groups (P = 0.542). Family history was positive in 26 patients who underwent excision. Of these, 9 (34.6%) had malignancy, while the others were benign, which was not statistically significant (P = 0.334). There was no statistically significant difference between the groups in terms of the presence of sonographic and MRI features (P = 0.547, P = 0.567, respectively). Of the patients with suspicious microcalcifications on mammography, 66.6% (8/12) were in the premalignant-malignant group, which was statistically significant (P = 0.001).
Radiologically, 17 of the lesions were BIRADS 4A, 38 were BIRADS 4B, 16 were BIRADS 4C and two were BIRADS 5. The BIRADS category and CNB results of those with malignant excision pathologies are shown in Table 2.
| BIRADS category | CNB pathology | Excision pathology |
|---|---|---|
| 4B (MG) | Fibroepithelial proliferative changes | DCIS |
| 4B (USG) | Adenosis | DCIS |
| 4B (MG) | Fibroepithelial proliferative changes | DCIS |
| 4B (MRI) | Fibroepithelial proliferative changes | Invasive ductal carcinoma |
| 4B (MRI) | Lobulite | Invasive ductal carcinoma |
| 4B (MRI) | Intraductal papillomatosis | DCIS |
| 4B (MRI) | Ductal hyperplasia | Microinvasive DCIS |
| 4B (MRI) | Intraductal papillomatosis | DCIS |
| 4B (USG) | Intraductal papillomatosis | DCIS |
| 4B (MRI) | Radial scar | DCIS |
| 4C (MRI) | Glandular and stromal structures | DCIS |
| 4C (MG) | Columnar cell changes | DCIS |
| 4C (MRI) | Granulation | Encapsule papillary carcinoma |
| 4C (MRI) | Apocrin metaplasia | Invasive ductal carcinoma |
| 4C (MRI) | Adenosis | Invasive ductal carcinoma |
| 4C (MRI) | Fibroepithelial lesion | Musinous carcinoma |
| 4C (MRI) | Fibroepithelial lesion | DCIS |
| 4C (MG) | Fibroepithelial proliferative changes | DCIS |
| 5 (MRI) | Papillary lesion | Encapsule papillary carcinoma |
Abbreviations: BIRADS, Breast Imaging Reporting And Data System; CNB, core needle biopsy; DCIS, ductal carcinoma in situ; MG, mammography; USG, ultrasonography; MRI, magnetic resonance imaging.
Lesions were grouped as 4A-4B and 4C-5. The BIRADS category 4C-5 was statistically significantly higher in the malignant group (P = 0.013). In univariable analyses considering malignancy as the dependent variable, age, presence of mammographic microcalcifications, and BIRADS category were statistically significant (P = 0.016, P = 0.002, P = 0.010, respectively). In multivariable analyses, BIRADS category and presence of microcalcifications were statistically significant (P = 0.029, P = 0.004, respectively) (Table 3).
| Variables | Malignant | Benign-borderline | Univariate b | Multivariate c | |||
|---|---|---|---|---|---|---|---|
| Values | Values | P-value d | OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Age (y); mean ± SD | 50.2 ± 9.0 | 42.1 ± 12.2 | 0.011 | 1.07 (1.01 - 1.12) | 0.016 | NS | - |
| Size (mm); median | 20 | 24 | 0.646 | NA | - | - | - |
| Family history | 0.334 | NA | - | NA | - | ||
| Yes | 9 (47) | 17 (32) | |||||
| No | 10 (53) | 37 (68) | |||||
| Breast composition | 0.542 | NA | - | NA | - | ||
| A - B | 7 (37) | 14(26) | |||||
| C - D | 12 (63) | 40 (74) | |||||
| BIRADS category | 0.013 | ||||||
| 4C-5 | 9 (47) | 9 (17) | 4.50 (1.42 - 14.22) | 0.010 | 4.04 (1.15 - 14.16) | 0.029 | |
| 4A-4B | 10 (53) | 45 (83) | 1 | 1 | |||
| Suspicious microcalcification | 0.001 | ||||||
| Yes | 8 (42) | 4 (7) | 9.09 (2.32 - 35.64) | 0.002 | 8.28 (1.98 - 34.59) | 0.004 | |
| No | 11 (58) | 50 (93) | 1 | 1 | |||
| Sonographic suspicious findings | 0.547 | NA | - | NA | - | ||
| Yes | 8 (42) | 29 (54) | |||||
| No | 11 (58) | 25 (46) | |||||
| Suspicious findings on dynamic MRI | 0.567 | NA | - | NA | - | ||
| Yes | 19 (100) | 50 (93) | |||||
| No | 0 (0) | 4 (7) | |||||
Abbreviations: OR, odds ration; NA, not available; NS, not significant; BIRADS, Breast Imaging Reporting and Data System; MRI, magnetic resonance imaging.
a Values are expressed as No. (%) unless otherwise indicated.
b Mantel-Haenszel common odds ration estimate.
c Logistic regression method backward stepwise (Wald).
d Chi-square or Fisher’s exact tests P-value.
5. Discussion
The radiologic-pathologic discordance rate has been reported between 2.2% and 6% in the literature (6, 7). However, open biopsy was performed in 119/667 (17.8%) of clinically or mammographically suspicious lesions in the study by Davidson et al. (8). In our study, the discordance rate after excision and 2-year follow-up was 1.9% (19/986). Our study showed that there was histopathologic discordance between CNB and excision in 26% (19/73) of the lesions. Liberman et al. (9) reported the overall malignancy rate as 24.4% in a study on imaging-histological discordance and concluded that discordance is an indication for immediate referral to surgery. In a study by Wan et al. in 2024, the discordance rate was found to be 30.9% (3). Davidson et al. showed that excision biopsies after CNB detected malignancy in 30% of cases (8). These rates are similar to our study. Subsequent EB revealed malignancy in up to 64% of discordant benign lesions obtained by image-guided CNB (10). In our study, older mean age was found to be significantly associated with malignant excision results, similar to Wan et al. (3). It is generally accepted that breast cancer is more common in older people. Age was not found to be significant in the study of Jung et al. only in BIRADS 3 category lesions. We think that the reason for this is that the majority of the lesions were benign (11). Positive family history was not different between the groups (P = 0.334). Family history was present in 47.4% (9/19) of patients who were found malignant by excision.
A higher BIRADS category was statistically significantly associated with malignancy at excision. When the lesions were classified according to BIRADS category, it was observed that the rate of malignancy detection increased from BIRADS 4A to BIRADS 5, in accordance with the literature. In our study, after excision, no malignancy was detected in BIRADS 4A lesions; 26.3% of 4B lesions, 50% of 4C lesions, and 50% of BIRADS 5 lesions were found malignant. Soyder et al. reported a carcinoma upgrade rate of 20% in the BIRADS 4 category and 53.8% in the BIRADS 5 category (6). Suspicious findings in terms of malignancy detected by ultrasound and MRI used in BIRADS categorization were not significantly different between the groups histopathologically. On the other hand, the presence of microcalcifications was significantly higher in the group with malignant excision results. In the study by Wan et al., the relationship between the presence of suspicious microcalcifications and discordance was investigated but not found to be significant (3). The reason for this is that there is no possibility of stereotactic biopsy in mammography in our hospital; the projection of microcalcifications is marked on the skin with a pencil on mammography, and an ultrasound-guided biopsy is performed from this area. This increases the possibility of failure and leads to discordant benign lesions. There were no significant differences in tumor size and breast composition between the groups (P = 0.646, P = 0.542, respectively). Contrary to expectations, tumor size was slightly higher in the benign group than in the malignant group. This may be due to the fact that the benign group has larger lesions with cystic components instead of pure solid lesions.
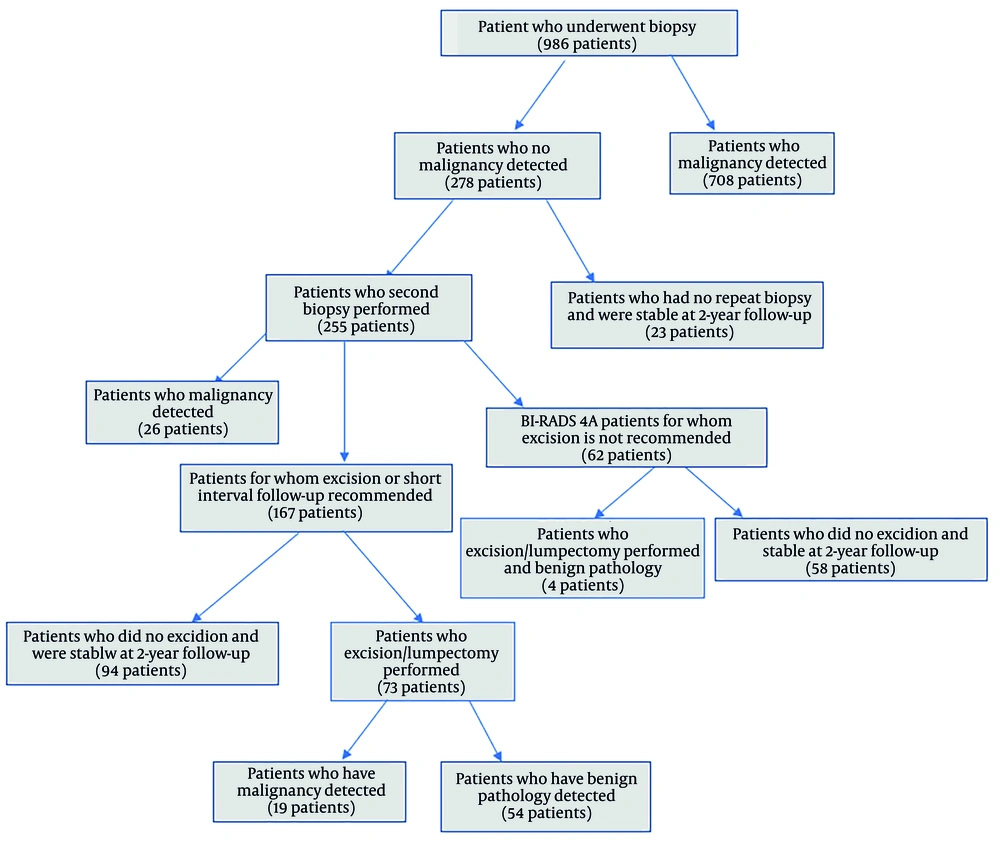
In addition to its high diagnostic efficiency, CNB has some disadvantages. The most important one is the difficulty of interpretation due to the small size of the sample, fragmentation, and distortion. Additionally, some benign proliferative, borderline, and in situ lesions, as well as papillary lesions, are challenging in terms of diagnosis (12, 13). Atypical ductal proliferation with high or intermediate nuclear grade in an intraductal papilloma qualifies as ductal carcinoma in situ (DCIS) regardless of size (14). In our study, a total of 14 papillary lesions were found after excision, of which 2 (14%) were encapsulated papillary carcinoma (EPC), 3 (21%) were ductal papillomatosis including DCIS and 9 (65%) patients had intraductal papilloma. One of the two lesions defined in the BIRADS 5 category was diagnosed as EPC after excision, while one of the two lesions was interpreted as a papillary lesion by CNB (Figure 2). In the literature, there are studies suggesting that papillary lesions detected by CNB can be safely followed up (15, 16), but we think that such lesions should be referred for excision because of the uncertain malignancy potential.
On contrast-enhanced magnetic resonance imaging (MRI), T1-weighted pre-contrast images (A) demonstrate an iso- to hypointense lesion located in the retroareolar region (black arrow). Post-contrast T1-weighted images (B) reveal heterogeneous peripheral enhancement within the solid component of the lesion (black arrow). T2-weighted images (C) show an iso- to hyperintense lesion containing both solid and cystic areas (black arrow). Diffusion-weighted imaging (D) indicates diffusion restriction within the solid portion (black arrow). A core needle biopsy (CNB) classified the lesion as a papillary neoplasm with a Breast Imaging Reporting and Data System (BI-RADS) category 5 rating. Subsequent surgical excision confirmed the diagnosis of encapsulated papillary carcinoma (EPC).
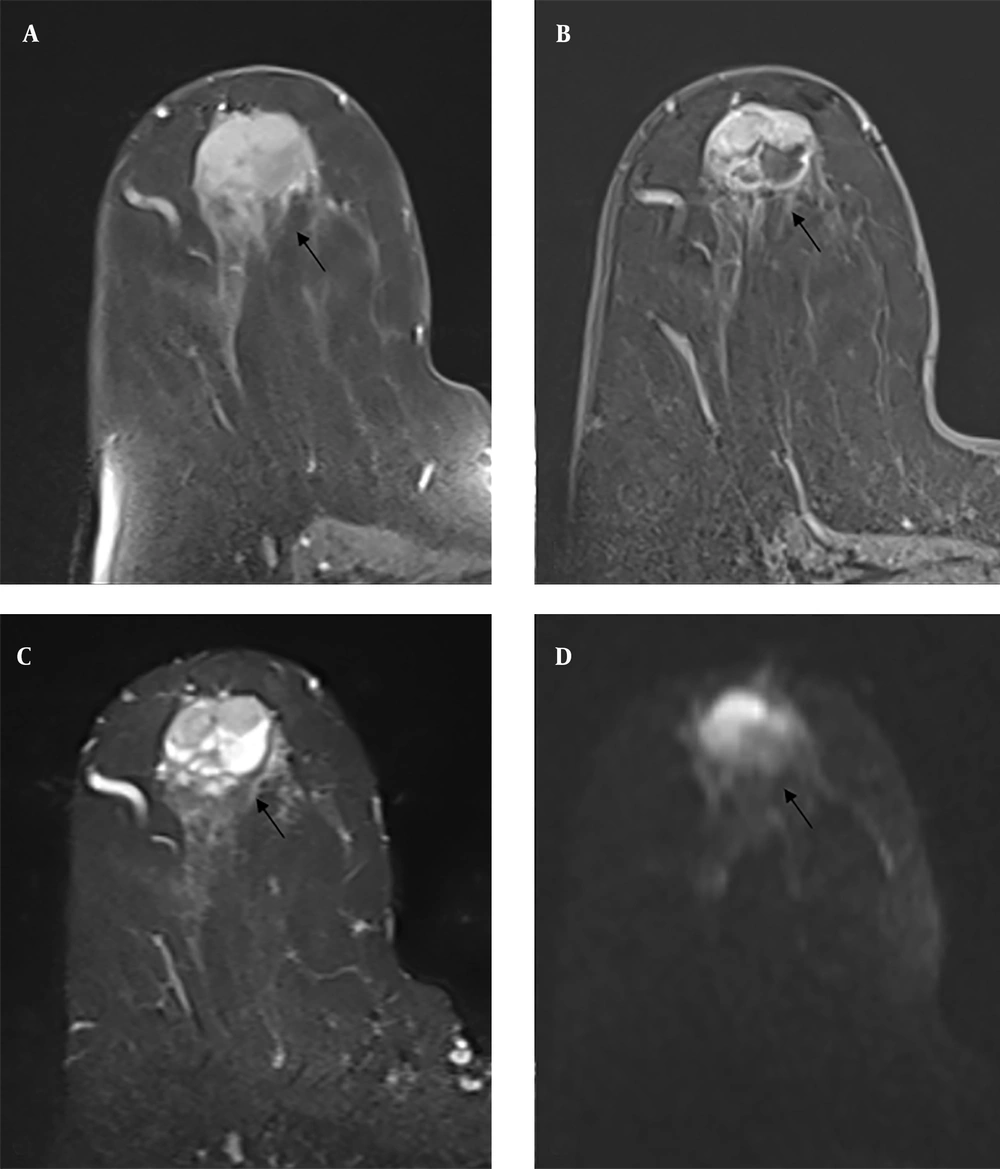
In the other BIRADS 5 lesions in our study, both CNB and excision resulted in fat necrosis. The lesion with fat necrosis is shown in Figure 3. Fat necrosis consists of solid hypoechoic lesions causing tissue distortion and shadowing posteriorly on ultrasound, which may be radiologically confused with malignancy. On mammography, coarse calcifications, amorphous, linear, fine branching, and pleomorphic microcalcifications, as well as asymmetry, may be observed (17). In a radiological and clinical study by Bilgen et al. on fat necrosis, increased echogenicity of subcutaneous tissue was observed in 26.9% of lesions, anechoic masses with posterior acoustic shadowing in 16.6%, solid masses in 14.2%, and cysts with internal echoes in 11.1%, regardless of the presence of small cysts (18). It has a wide range of findings on MRI and may mimic malignancy. The most common finding is round or oval hypointense masses on T1W fat-suppressed images (19). After intravenous administration of contrast media, it may show highly variable contrast enhancement patterns and kinetic analysis (20).
Mammography shows irregularly circumscribed asymmetric opacity increase (A, B) in the upper inner quadrant. On contrast-enhanced magnetic resonance imaging (MRI), the irregularly circumscribed heterogeneously contrasted lesion (C, D) with a type 1 time-signal intensity curve on post-contrast T1-weighted (T1W) images was evaluated in Breast Imaging Reporting and Data System (BIRADS) 5 categories with mammography and MRI findings. Both core needle biopsy (CNB) pathology and excision pathology results were reported as fat necrosis.
In a patient with a borderline phylloid tumor as a result of excision, the CNB result was interpreted as fibroadenoma. There are no reliable radiologic features that can distinguish a phylloid tumor from a fibroadenoma. On ultrasound, it is frequently seen as well-circumscribed solid masses with a heterogeneous internal structure. However, a phylloid tumor may be suspected in rapidly growing lesions larger than 4 cm (21). On MRI, it is usually T1 hypointense and T2 homogeneous hypointense/hyperintense. In post-contrast series, the kinetic curve pattern may be type 1 or type 2 (22).
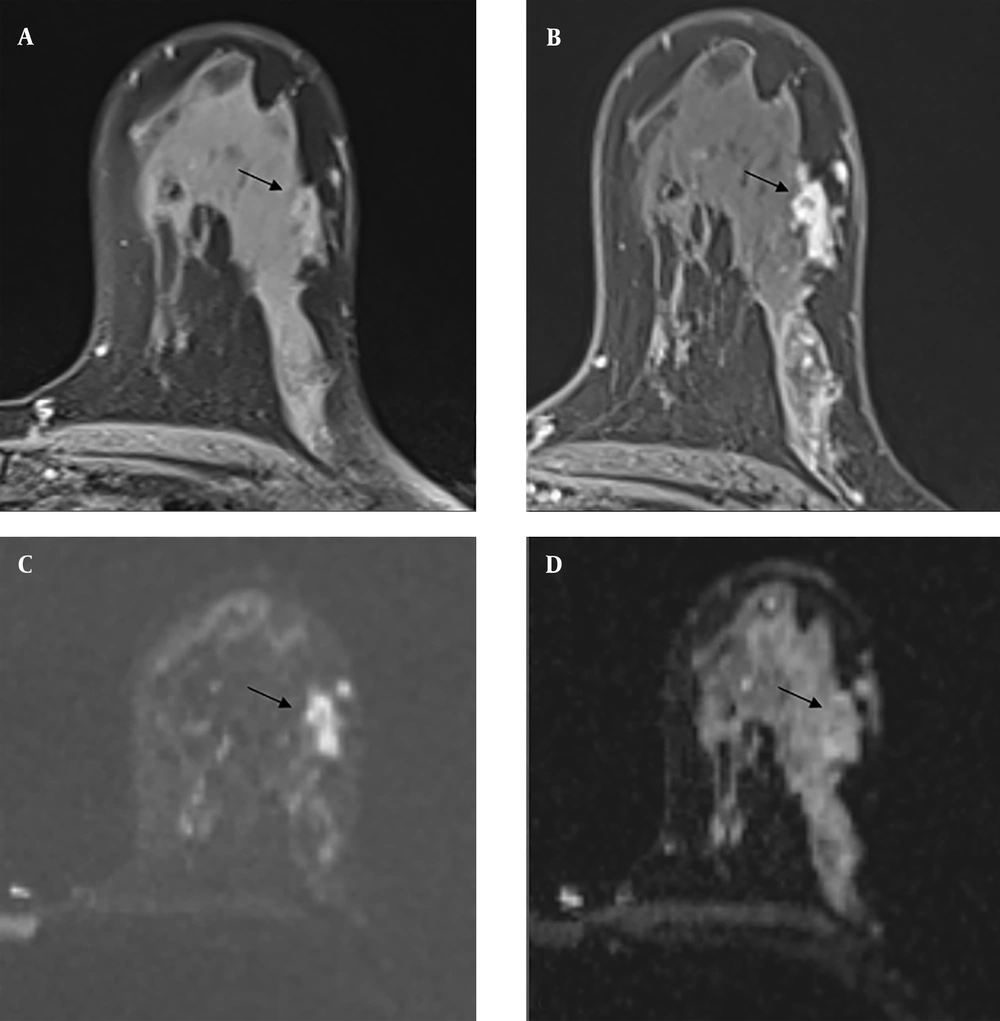
The three lesions with radial scars detected by biopsy were radiologically defined as BIRADS category 4B at the time of diagnosis, and 1 of them (33%) was found to have DCIS after excision (Figure 4). Radial scars are benign breast lesions of unknown clinical significance that are detected with increasing frequency in women undergoing breast cancer screening (23). Invasive carcinoma was found in 11% of patients with radial scars in a study by Davidson et al. (8).
Contrast-enhanced magnetic resonance imaging (MRI) of a Breast Imaging Reporting and Data System (BIRADS) 4B category lesion with a radial scar on core needle biopsy (CNB) pathology and ductal carcinoma in situ on excision pathology. A mild hyperintense lesion is seen on pre-contrast T1-weighted (T1W) images (A), and a homogeneously contrasted irregularly circumscribed lesion is seen on post-contrast T1W images (B) (black arrows). It is shown to be hyperintense on diffusion-weighted imaging (C) and hypointense on apparent diffusion coefficient images (D) due to diffusion restriction. (black arrows)
Some limitations of our study should be mentioned. The retrospective design of the study and the small number of patients can be considered the most important ones. Therefore, the generalizability of the results is limited. Additionally, confounding factors such as the menstrual cycle and hormone use may affect imaging results, especially MRI. Since vacuum biopsy is not covered by social insurance in our country, we do not have the possibility of performing vacuum biopsy. This situation reduces the possibility of making a correct diagnosis before excision. Furthermore, the ultrasound-guided biopsy technique is operator-dependent and causes individual differences. The diagnostic sensitivity of fragmented specimens is reduced. On the other hand, although histopathological evaluation by a single pathologist provides standardization, it may cause diagnostic errors in some lesions that are open to interpretative differences.
In conclusion, the BIRADS system is reliable in determining the malignancy risk of breast masses radiologically. In breast masses where malignancy is suspected based on clinical and imaging findings, CNB is a reliable method with high diagnostic accuracy. However, additional surgery is required when there is clinical and radiologic discordance with the CNB findings. Excision of papillary lesions is necessary to confirm CNB findings and exclude possible malignancy. Multicenter prospective studies in larger populations are necessary to confirm the findings.