1. Background
The computed tomography (CT)-guided percutaneous thermal ablation techniques, including microwave ablation (MWA) and radiofrequency ablation (RFA), have increasingly been considered as curative clinical surgical techniques that may replace surgical resection. Moreover, they have gradually been used for patients with tumor recurrence who require re-treatment and for malignant tumor patients who are not suitable for surgical treatment clinically (1-3). The treatment efficacy of MWA and RFA has been well verified in clinical practice. However, MWA has more advantages compared to RFA, including higher microwave ablation power, which can significantly increase the local temperature, shorter ablation time required for tumors of the same diameter, and less influence by the cooling effect caused by blood perfusion. For tumors close to blood vessels and prone to the heat-sink effect, it can also achieve relatively uniform inactivation (4).
The most basic operation of MWA is the percutaneous puncture technique. Although percutaneous puncture is a minimally invasive and safe operation technique, there are still some deficiencies in the treatment of liver malignancies in dangerous areas, such as high treatment difficulty, uncertain efficacy, and a relatively low safety coefficient. Thermal ablation of liver malignancies adjacent to the diaphragm, gastrointestinal tract, subcapsular liver, large blood vessels, and beside the gallbladder is prone to cause serious complications such as incomplete ablation, tumor rupture, implantation, hemorrhage, and thermal injury to adjacent tissues (5). Especially for tumors around the heart, not only during the puncture process but also during the ablation process, it may lead to serious complications such as heart damage and even clinical death (6).
Currently, there are many studies on the safety and efficacy of ablation treatment for lesions such as subcapsular liver, adjacent to the diaphragm, gallbladder, and large blood vessels (7, 8). However, there are few studies on CT-guided MWA for liver malignancies adjacent to the heart. One study evaluated the safety and efficacy of percutaneous hepatic MWA near the heart (distance to myocardium = 1.1 ± 1.1 cm). Among 118 ablations, 27 with ablation zones close to the myocardium formed the study group. Periprocedural imaging and medical records were used. After a median 13.6-month follow-up, no arrhythmias occurred. There were no differences in key parameters. It concludes that such ablation near the heart may be safe and effective (9).
Another retrospective study evaluated the therapeutic effect of MWA on hepatocellular carcinomas (HCC) with a diameter of less than 4 cm located in difficult positions. A total of 81 patients were retrospectively analyzed in this study. Among them, 43 patients were assigned to the difficult position group, including those with tumors adjacent to the heart, with a distance of ≤ 5 mm. After an average follow-up period of 3.4 months, no serious complications occurred. There were no differences found between the two groups in terms of therapeutic effect, local tumor progression, and complication rate (10).
2. Objectives
Based on the above research results, we consider that MWA can also be performed on hepatic malignant tumors adjacent to the heart, and the safety should be relatively high. Thus, this study aims to investigate the feasibility and safety of CT-guided MWA for treating hepatic malignant tumors adjacent to the heart, summarize the short-term curative effects of the treatment, and conduct a comparative study with microwave ablation of hepatic malignant tumors in other locations.
3. Patients and Methods
3.1. General Information
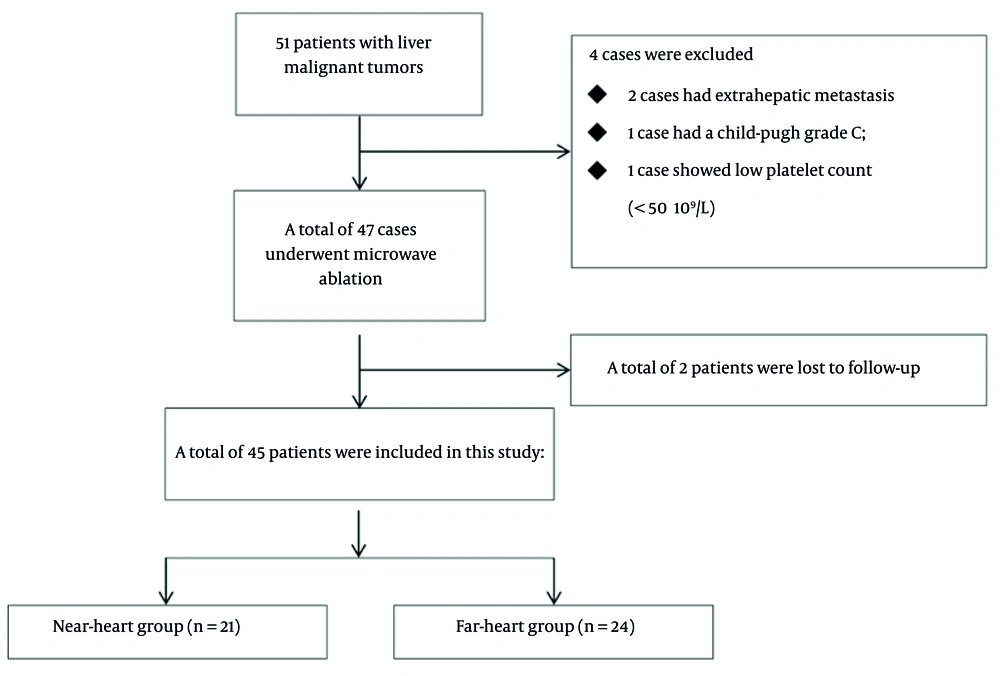
A total of 51 patients with liver malignant tumors, scheduled to undergo CT-guided percutaneous MWA treatment at the First Hospital of Qinhuangdao from July 2016 to June 2020, were collected for this single-center study. All patients received medical treatment at our center. The inclusion criteria were as follows: (1) Confirmed by puncture biopsy or diagnosed as primary liver cancer or metastatic liver cancer by imaging; (2) the maximum diameter of a single nodule ≤ 5 cm, while the maximum diameter of multiple nodules ≤ 3 cm, and the number of tumors does not exceed 3, as smaller tumors are more likely to achieve complete ablation and have relatively fewer complications; (3) liver function Child-Pugh grade is A or B, indicating that the patients' liver function is in a relatively good state, enabling them to tolerate the MWA and the potential stress reactions it may bring about; (4) platelet count ≥ 50 × 109/L.
Exclusion criteria were as follows: (1) Vascular invasion or extrahepatic metastasis; (2) liver function Child-Pugh grade is C; (3) refractory ascites; (4) uncorrectable coagulation disorder, platelet count < 50 × 109/L; (5) severe cardiopulmonary failure. According to the tumor location, the patients were divided into the near-heart group and the far-heart group. Referring to reports in previous literature (8), those with the vertical distance between the tumor and the heart measured by CT or magnetic resonance imaging (MRI) ≤ 2 cm were classified as the near-heart group, and those > 2 cm were classified as the far-heart group. All patients included in the study signed the informed consent form. The detailed research flowchart can be found in Figure 1.
A total of 45 patients were included as shown in the flowchart, with 21 cases in the near-heart group and 24 cases in the far-heart group. There were 28 males and 17 females, aged 45 - 78 years, with an average age of 60.44 ± 10.16 years. There was no statistical difference in basic data such as age, gender composition ratio, average number of tumors per person, maximum tumor diameter, tumor category, ablation power, and ablation time between the two groups. The detailed clinical data of the two groups are shown in Table 1.
| Group | Near-heart group (n = 21) | Far-heart group (n = 24) | Statistic | P-value |
|---|---|---|---|---|
| Age | 60.29 ± 10.42 | 60.58 ± 10.15 | t = 0.097 | 0.923 |
| Gender (male/female) | 15/6 | 13/11 | χ2 = 1.420 | 0.233 |
| Average number of tumors per person | 1.76 ± 0.83 | 1.63 ± 0.77 | t = 0.574 | 0.569 |
| 1 | 10 | 13 | χ2 = 0.367 | 0.833 |
| 2 | 7 | 8 | ||
| 3 | 6 | 5 | ||
| Total number of tumors | 37 | 39 | - | - |
| Maximum tumor diameter (mm) | 21.70 ± 8.53 | 19.54 ± 8.57 | t = 0.574 | 0.274 |
| Tumor category (primary/secondary) | 16/5 | 18/6 | χ2 = 0.009 | 0.926 |
| Hepatocellular carcinoma | 13 | 16 | χ2 = 0.403 | 0.817 |
| Intrahepatic cholangiocarcinoma | 3 | 2 | ||
| Liver metastasis of colon cancer | 5 | 6 | ||
| Child-Pugh A/B | 15/6 | 16/8 | χ2 = 0.118 | 0.731 |
| Ablation power (W) b | 50 ± 5 | 50 ± 5 | Z = -0.843 | 0.415 |
| Ablation time (min) | 6.65 ± 2.53 | 6.00 ± 2.35 | t = 1.159 | 0.250 |
| Previously received systemic treatment (yes/no) | 16/5 | 18/6 | χ2 = 0.009 | 0.926 |
| Previously received local treatment for liver tumors (yes/no) | 18/3 | 21/3 | χ2 = 0.031 | 0.860 |
a Values are expressed as mean ± SD.
b Only three ablation powers, namely 45, 50 and 55 W, were used for the ablation power.
3.2. Instruments and Methods
The MWA treatment utilized a microwave ablation system (Nanjing ECO Medical Technology Co.) therapeutic apparatus with a frequency of 2450 MHz and an output power of 40 - 60 W, and a 16G cold-circulating monopolar needle (length 15 cm). Microwave ablation was performed under CT guidance using a revolution CT (64 slices, General Electric Company HealthCare). After local anesthesia with 1% lidocaine or pain relief treatment with morphine hydrochloride injection, the microwave electrode was implanted by puncture under CT guidance. According to the shape, size, and position of the tumor, the microwave output power and time were adjusted. The ablation time was generally 5 - 10 minutes. The ablation range was designed to be as large as possible, preferably 5 mm larger than the tumor edge. During the ablation process, no water isolation or other thermal protection techniques were used. For tumors with a maximum diameter of 3 - 5 cm and a rich blood supply, transcatheter arterial embolization was performed before MWA treatment to reduce the influence of the heat-sink effect.
3.3. Efficacy Evaluation
Both groups of patients underwent enhanced liver MR examinations at 1 month, 3 months, 6 months, 12 months, and within 3 years as needed after MWA treatment. The follow-up after surgery was based on imaging findings to evaluate the efficacy of MWA treatment and calculate the complete ablation rate and local tumor progression rate. Complete ablation was defined as the non-enhanced area in the contrast-enhanced MR image one month after the ablation procedure completely covering the scope of the tumor [Enhanced MR parameters: Plane: Axial, Time of repetition (TR)/time of echo (TE): 4.44/2.16, angle (°): 10, slice thickness (mm): 3, field of view (FOV) (mm2): 380, voxel size (mm3): 1.2 × 1.2 × 3.0, delay (s): 0, 25, 60, 180, scan time (s): 17]. The complete ablation rate was calculated as the number of completely ablated lesions divided by the total number of lesions, multiplied by 100%.
Tumor progression after treatment was observed. Local tumor progression was defined as the appearance of a new abnormal enhanced lesion in the ablation area or in a position adjacent to the ablation area during the follow-up process. The local progression rate was calculated as the number of local progression lesions divided by the total number of lesions, multiplied by 100%. The progression-free survival rate and overall survival rate of both groups of patients were calculated.
Microwave ablation complications were observed and recorded, especially the electrocardiogram and myocardial enzyme indexes. Procedure-related complications were graded according to the Society of Interventional Radiology (SIR) classification system. Serious complications were defined as those that may endanger the patient's life safety and require hospitalization or an extended hospital stay (SIR C-F). The incidence of serious complications was calculated.
3.4. Statistical Analysis
Statistical analysis was conducted using SPSS (IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp.). Normally distributed measurement data were represented by mean ± SD. The comparison between two groups was performed using an independent sample t-test. Measurement data that did not conform to a normal distribution were represented by the median (interquartile range), and the comparison between two groups was performed using a rank sum test. Counting data were represented by a percentage, and the comparison between two groups was performed using a χ2 test or Fisher's exact probability method.
The Kaplan-Meier method was used to analyze the local tumor progression rate, progression-free survival rate, and overall survival rate, with comparisons between two groups performed using a log-rank test. Cox regression analysis was performed on all 45 patients included in this study to examine the influence of multiple research factors on the overall survival rate. A P-value of < 0.05 indicated a statistically significant difference.
4. Results
4.1. Comparison of Complete Ablation Rate and Local Tumor Non-progression Between the Two Groups
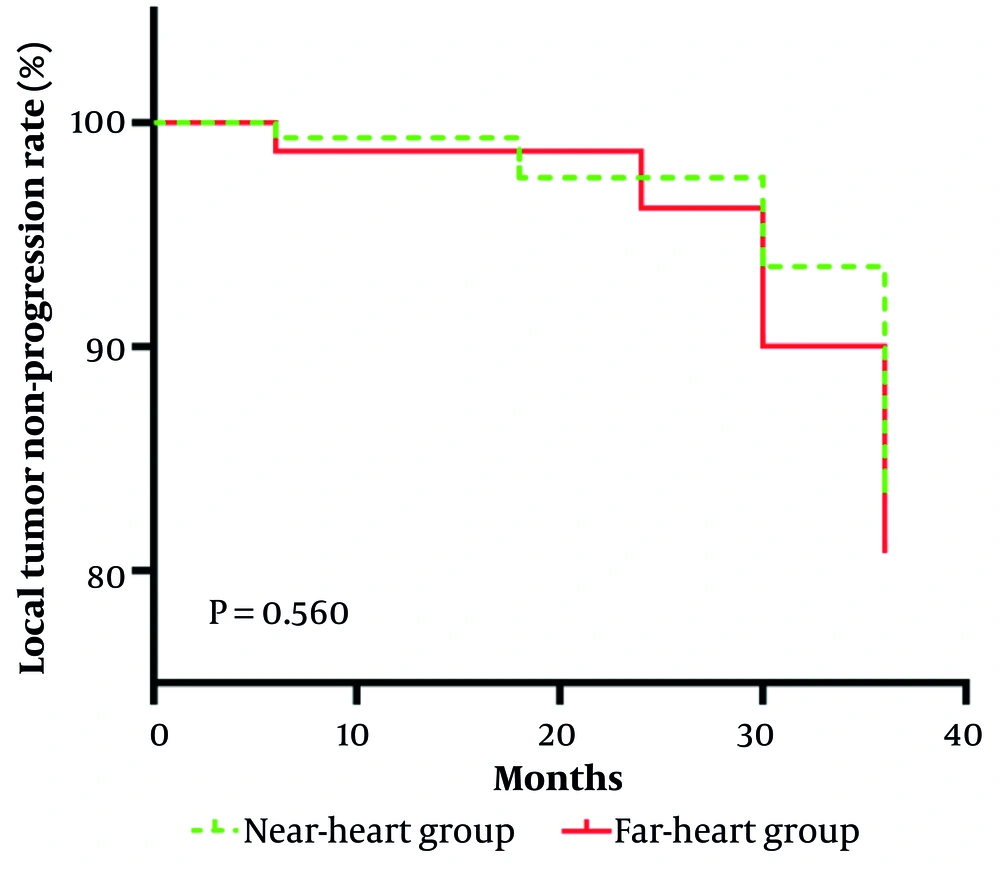
The complete ablation rates of both groups were 100%, and the difference was not statistically significant. The comparison of the local tumor non-progression rate between the two groups showed no statistically significant difference (χ2 = 0.3391, P = 0.560), as shown in Figure 2. There are two typical cases, which can be referred to in Figure 3.
This is a Kaplan-Meier curve showing the local tumor non-progression rate over time (in months) for the near-heart group and the far-heart group. Initially, the local tumor-progression rate for both groups was 100%, and then it gradually decreased with different trends. Log-rank test yielded a P-value of 0.560, indicating no significant difference in local tumor-progression rate between the near-heart group and the far-heart group.
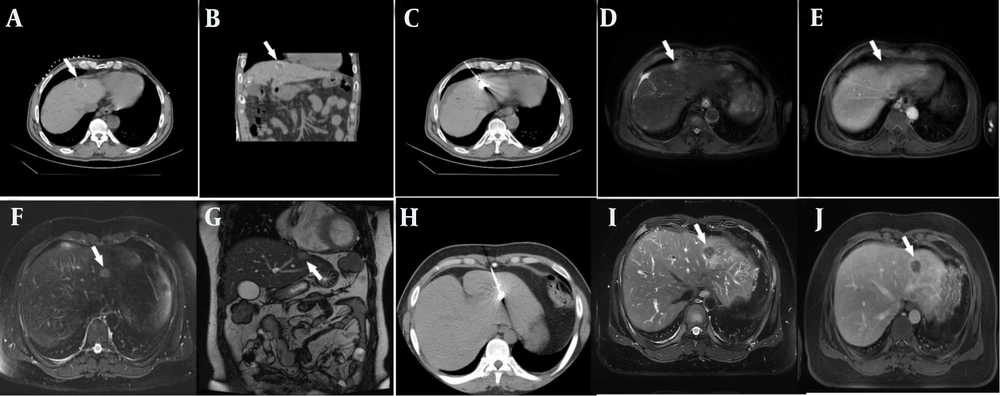
Two typical patients. A and B, depict a 55 - year - old male patient with a history of hepatitis C. He was diagnosed with primary liver cancer two months ago and had previously undergone transcatheter arterial chemoembolization (TACE). This time, computed tomography (CT)-guided microwave ablation was performed for the treatment of liver malignant tumor. The vertical distance from this lesion to the heart is 1.5 cm. C shows the CT scan image during the microwave ablation process of this patient. D and E respectively show the abdominal magnetic resonance T2WI and T1WI enhanced images during the one - year re-examination after ablation, indicating tumor inactivation. F and G show a 34-year-old male patient with liver metastases from colon cancer. The vertical distance from the intra - hepatic lesion to the heart is 0.5 cm, F: Magnetic resonance T2WI, G: Magnetic resonance coronal t2 haste. H shows the CT scan image during the microwave ablation process of this patient. I and J respectively show the abdominal magnetic resonance T2WI and T1WI enhanced images during the one - year re-examination after ablation, indicating tumor inactivation. The area pointed by the white arrow is the lesion in the liver. Both of these cases show that the tumors in the liver near the heart have achieved the effect of complete ablation, and there has been no tumor recurrence during the three-year follow-up period (the location of the intrahepatic tumor is indicated by the white arrows).
4.2. Comparison of Progression - Free Survival Rate Between the Two Groups
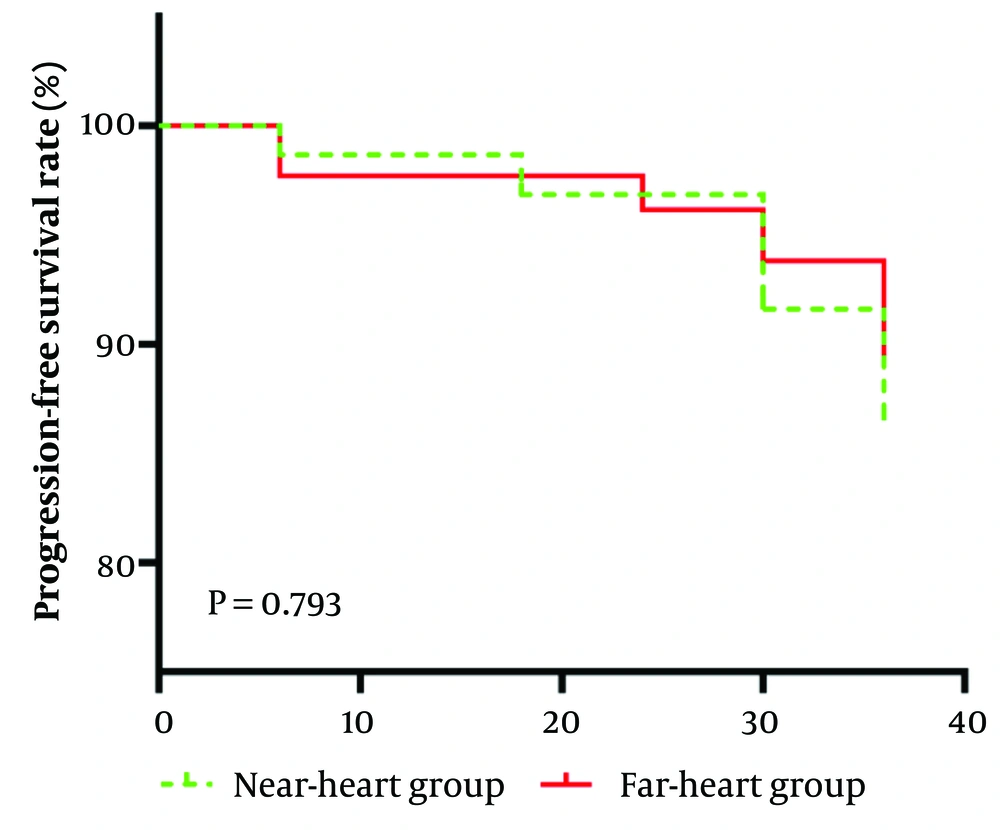
The comparison of the progression-free survival rate between the two groups showed no statistically significant difference (χ2 = 0.069, P = 0.793), as shown in Figure 4.
This is a Kaplan-Meier curve depicting the progression-free survival rate over time for the near- heart group and the far-heart group. The y-axis shows the progression-free survival rate (%), and the x-axis shows time. Initially, both groups had a progression-free survival rate of 100%. As time went on, the rates for both groups decreased. A log-rank test was conducted, and the resulting P-value was 0.793, indicating that there is no significant difference in the progression-free survival rate between the near-heart group and the far-heart group.
4.3. Comparison of Overall Survival Rate between the Two Groups
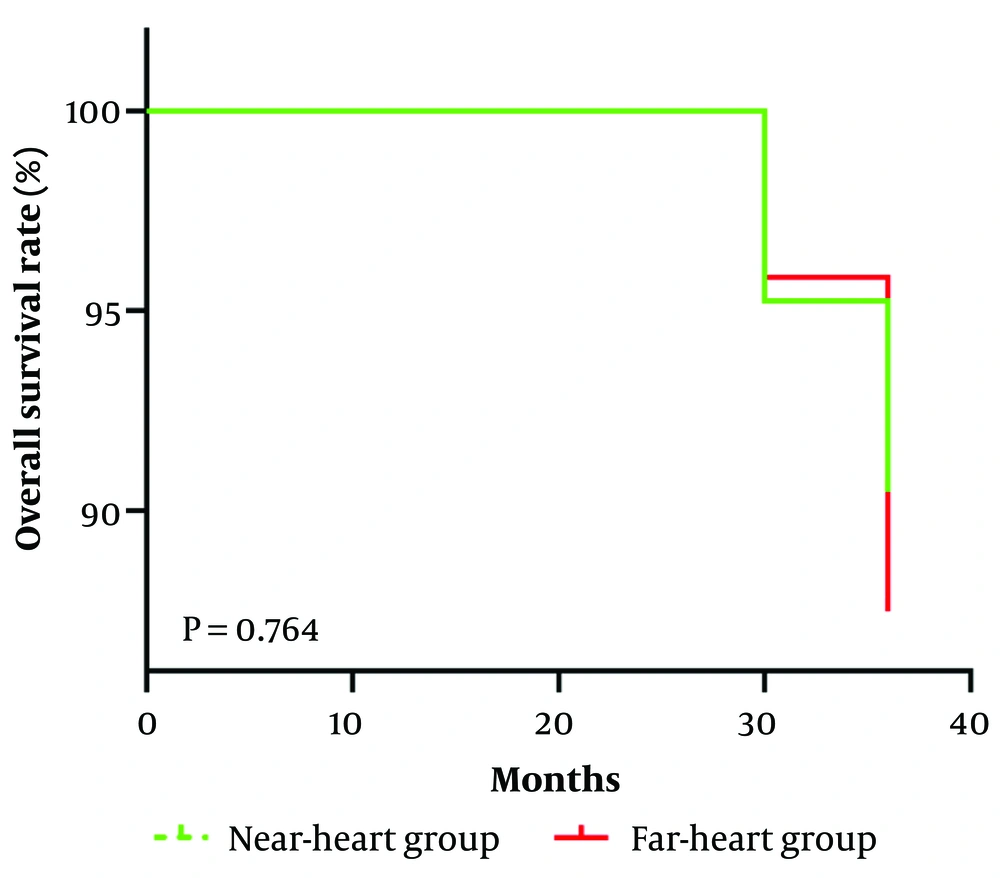
The comparison of the overall survival rate between the two groups showed no statistically significant difference (χ2 = 0.090, P = 0.764), as shown in Figure 5.
This is a Kaplan-Meier curve illustrating the overall survival rate over time for the near-heart group and the far-heart group. The y-axis represents the overall survival rate (%), while the x-axis represents time in months. Initially, the overall survival rate for both groups was 100%. After approximately 30 months, the rates for both groups began to decline. A log-rank test was performed, resulting in a P-value of 0.764, which indicates that there is no significant difference in the overall survival rate between the near-heart group and the far-heart group.
4.4. Comparison of Incidence of Serious Complications Between the Two Groups
No cardiac function abnormality occurred in either group. The serious complications that occurred in both groups were subcapsular hemorrhage. The incidence of serious complications in the near-heart group was 4.8% (1/21), and in the far-heart group was 4.2% (1/24). After active intravenous hemostasis treatment, both improved without the need for surgical treatment. The comparison of the incidence of serious complications between the two groups showed no statistically significant difference (χ2 = 0.009, P = 0.923), with a hazard ratio (HR) of 1.15 and a 95% confidence interval (CI) of (0.0675, 19.6033). The Cox regression results are shown in Table 2.
| Variables | HR | 95% CI | P-value |
|---|---|---|---|
| Age | 0.899 | 0.585 - 1.382 | 0.682 |
| Gender | |||
| Female | 1 | ||
| Male | 1.13 | 0.465 - 2.740 | 0.787 |
| Number of tumors per person | |||
| 1 | 1 | ||
| 2 | 1.039 | 0.976 - 1.117 | 0.295 |
| 3 | 1.371 | 0.338 - 5.567 | 0.659 |
| Maximum tumor diameter (mm) | 1.601 | 0.889 - 2.892 | 0.116 |
| Tumor category | |||
| Hepatocellular carcinoma | 1 | ||
| Intrahepatic cholangiocarcinoma | 1.103 | 0.381 - 3.205 | 0.861 |
| Liver metastasis of colon cancer | 1.182 | 0.393 - 3.528 | 0.773 |
| Child-pugh | |||
| A | 1 | ||
| B | 1.825 | 0.852 - 3.508 | 0.122 |
| Ablation power (W) | |||
| ≤ 50 | 1 | ||
| > 50 | 1.13 | 0.465 - 2.740 | 0.172 |
| Ablation time (min) | 0.783 | 0.515 - 1.193 | 0.255 |
| Previously received systemic treatment | |||
| Yes | 1 | ||
| No | 1.457 | 0.831 - 2.552 | 0.189 |
| Previously received local treatment for liver tumors | |||
| Yes | 1 | ||
| No | 0.981 | 0.558 - 1.723 | 0.946 |
Abbreviations: HR, hazard ratio; CI, confidence interval.
a All data in this table are based on the analysis of 45 patients.
5. Discussion
Local thermal ablation treatment technology is one of the radical treatment means for early liver malignancies. It is a minimally invasive surgery with the advantages of small trauma, fast recovery, and simple and easy operation. Hospitals capable of performing local ablation surgery for liver malignancies can offer this treatment, and it has a very wide range of application prospects (11). Moreover, MWA under image guidance has the characteristics of fast heating, less influence by the heat-sink effect, large ablation range, minimally invasive nature, and repeatability. Compared to surgical operations, it can retain more residual liver volume (12).
The image guidance methods can include B-ultrasound, CT, and MRI. MWA under B-ultrasound guidance has the advantages of real-time imaging, accurate guidance, simple operation, convenient movement, low cost, no radiation damage, and high energy efficiency (13). However, due to the difficulty in displaying some special-part lesions by B-ultrasound, resulting in inaccurate positioning, it may lead to incomplete ablation. The MWA under MRI guidance has the advantages of no radiation, high soft tissue resolution, and the ability to image in multiple directions and angles (14). However, due to high equipment requirements, high cost, and the tendency to produce motion artifacts, its development in clinical practice has been limited, and it has certain limitations (15).
The CT-guided MWA has good spatial resolution and can accurately determine whether the ablation needle is inserted into the center area of the tumor. This interventional technology is already very mature (16). Although CT-guided MWA is generally recognized as a very safe treatment method, some complications may still occur. When ablating lesions in high-risk areas, thermal ablation often leads to serious complications such as incomplete ablation, hemorrhage, tumor rupture, and thermal injury to surrounding tissues and organs. There have been previous reports that MWA for tumors adjacent to the gallbladder may easily lead to gallbladder perforation or acute cholecystitis (17), and studies have also found that when ablating lesions adjacent to the diaphragm, it may lead to pneumothorax or diaphragm damage, followed by shoulder pain and other symptoms (5). However, there are few studies on microwave ablation treatment for liver malignancies around the heart.
In this study, MWA treatment for lesions close to the heart can achieve the same efficacy as those not close to the heart. The 2-year progression-free survival rate can be maintained above 90%, making it a very effective treatment method. Moreover, the difference in the incidence of serious complications between MWA treatment for lesions close to the heart and those not close to the heart is not statistically significant, indicating that CT-guided MWA for tumors adjacent to the heart has high safety. Previously, Johnson et al. performed MWA treatment on 22 cases of liver malignancies below the heart, and the results showed that the technical success rate could reach 100%, with the incidence of serious complications being only 4.5%, which included severe chest pain and shoulder pain after surgery that improved significantly after pain relief treatment. The 1-year local tumor progression rate was 13.6%, and the 2-year local tumor progression was only 22.7% (6). This is consistent with our research results, indicating that even for lesions close to the heart, the safety of MWA treatment is still high, and the disease control efficacy is very good. This study also has certain limitations. First, the sample size is small. A small sample may increase the sampling error and reduce the accuracy of the research results. Second, this is a retrospective study, which makes it difficult to control some confounding factors, and there may be deviations in the integrity and accuracy of the data. Finally, the follow-up period of this study is short. The follow-up time may not be sufficient to capture the long-term recurrence of the disease, which could make the research results overly optimistic and overestimate the treatment effect, failing to provide comprehensive and accurate information for clinical practice. Based on these limitations, further improvements are needed in future research.
Due to the above-mentioned limitations, based on the existing research results, we consider that CT-guided MWA shows certain signs of effectiveness and safety in the treatment of liver tumors close to the heart.
In conclusion, this research indicates that CT-guided MWA demonstrates notable effectiveness and safety profiles in the treatment of liver tumors situated in close proximity to the heart. This minimally invasive technique enables precise targeting of tumors while minimizing potential damage to surrounding healthy tissues and vital structures. Given its favorable outcomes, MWA should be seriously considered as a viable and valuable treatment option for managing such challenging liver tumors.