1. Background
1.1. A General Overview of Large Language Models in Radiology
Large language models (LLMs) mark a key moment in artificial intelligence. They have major effects across many fields, especially in medical sciences (1). These models use large datasets and smart algorithms. They show great language accuracy and fluency, making their output sound like human speech (2, 3). The LLMs have demonstrated particular promise in radiology for patient triage, workflow optimization, and report generation (4, 5). The LLMs can automate the selection of imaging studies. This helps rank urgent cases, streamlining workflow and improving departmental efficiency (6, 7). Given the ongoing shortage of radiologists, LLMs could play a supportive role in these areas (8). A major drawback of LLMs in clinical workflows is their risk of creating inaccurate or inappropriate information. This can result in diagnostic errors and put patient safety at risk (9).
A core aspect of radiology practice involves choosing the appropriate imaging modalities and protocols to ensure precise diagnoses and enhance patient outcomes (10). Previous studies underscore the utility of LLMs in recommending imaging studies across various clinical scenarios, often using established standards like the American College of Radiology Appropriateness Criteria (ACR-AC) (11, 12). Rau et al. developed accGPT, a specialized chatbot based on ChatGPT-3.5-turbo, designed to provide personalized imaging recommendations consistent with ACR-AC. In a comparison involving 50 clinical scenarios, accGPT demonstrated superior accuracy and efficiency compared to radiologists and ChatGPT-3.5 and ChatGPT-4 (11). Similarly, Zaki et al. compared Glass AI and ChatGPT across 1,075 cases from ACR panels. Glass AI significantly outperformed ChatGPT (mean scores 2.32 ± 0.67 vs. 2.08 ± 0.74, P = 0.002), notably in polytrauma, breast, and vascular imaging, though both tools showed limitations in neurologic, musculoskeletal, and cardiac imaging panels (12).
1.2. The Specific Role of Magnetic Resonance Imaging Acquisition Protocols and Turkish Society of Radiology 2018 Magnetic Resonance Imaging and Computed Tomography Acquisition Standards Guideline
The Turkish Society of Radiology published the "Turkish Society of Radiology 2018 Magnetic Resonance Imaging and Computed Tomography Acquisition Standards Guideline" (TSR-2018 MCASG) in 2018. This guideline covers sequence selection, patient positioning, scanning parameters, and specific sequence requirements (13). However, given that national guidelines often differ across countries, testing LLM performance within the context of localized standards is crucial.
1.3. The Research Gap and Study Objectives
To our knowledge, no study has assessed the proficiency and knowledge of LLMs in determining magnetic resonance acquisitions and compared them to radiologists.
2. Objectives
This study aims to address this gap by evaluating the performance of various LLMs regarding TSR-2018 MCASG and comparing them with radiologists of different experiences.
3. Materials and Methods
3.1. Study Design
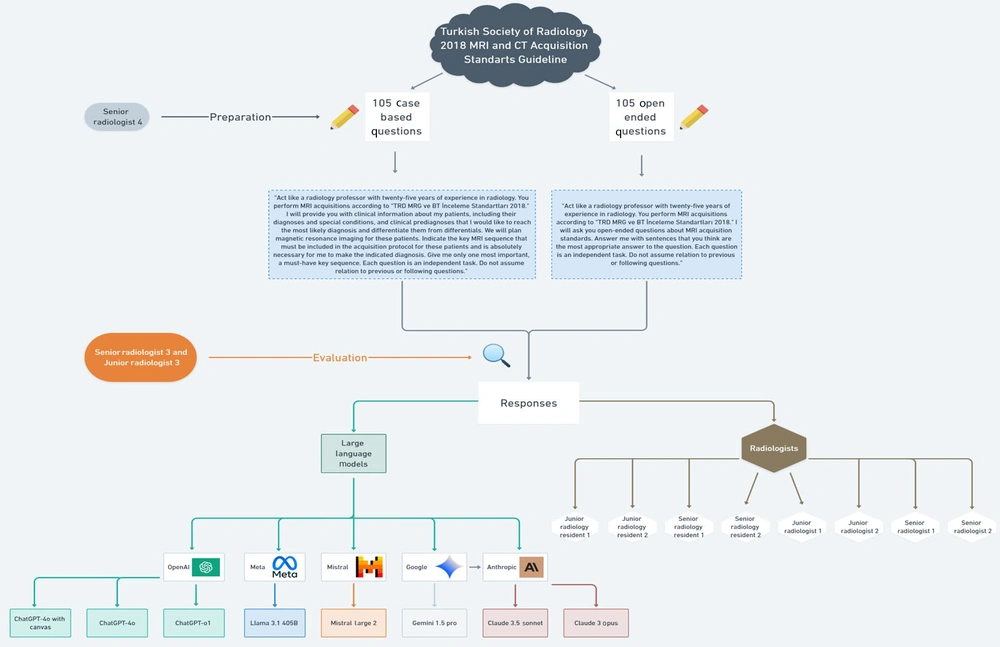
This cross-sectional observational study compares the performance of various LLMs — including ChatGPT-4o with canvas, ChatGPT-4o, ChatGPT-o1, Claude 3 Opus, Claude 3.5 Sonnet, Google Gemini 1.5 Pro, Meta Llama 3.1 405B, and Mistral Large 2 — with that of two junior radiology residents (JRRs), two senior radiology residents (SRRs), two board-certified [European Diploma in Radiology (EDiR)] junior radiologists (JRs), and two senior radiologists (SRs). The comparison focused on their proficiency regarding MRI acquisition standards and their ability to select the key MRI sequence for specific conditions. To address these abilities, open-ended questions (OEQs) and case-based questions (CBQs) were utilized, which were derived from TSR-2018 MCASG.
Since all questions and cases utilized and analyzed in this study are entirely fictional, no real patient data was used in this study. Also, there were no volunteers participating in this study. No patient information and images were used to eliminate the need for ethics committee approval. Therefore, ethical approval is not applicable for this study. The study methodology adhered to the Checklist for Artificial Intelligence in Medical Imaging (CLAIM) statement, ensuring transparency and reproducibility (14). An overview of the workflow is shown in Figure 1.
3.2. Participant Radiologists
1. Junior radiology residents: The JRR1 and JRR2 both have two years of experience in radiology and one year of experience in MRI.
2. Senior radiology residents: The SRR1 and SRR2 both have four years of experience in radiology and three years of experience in MRI.
3. Junior radiologists: JR1, JR2, and JR3 are all board-certified (EDiR) with seven years of experience in radiology and six years of experience in MRI.
4. Senior radiologists: SR1, SR2, SR3, and SR4 all have twenty-three years of experience in radiology and twenty years of experience in MRI.
The background of the radiologists is provided in Table 1.
| Variables | Radiologists (name initials) | Radiology experience (y) | MRI experience (y) | Certification |
|---|---|---|---|---|
| JRR | JRR1 (S.E.E.); JRR2 (H.K.) | 2 | 1 | — |
| SRR | SRR1 (Y.Ö.); SRR2 (M.K.) | 4 | 3 | — |
| JR | JR1 (Y.C.G.); JR2 (T.C.); JR3 (E.Ç.) | 7 | 6 | Board-certified (EDiR) |
| SR | SR1 (S.D.); SR2 (R.S.Ö.); SR3 (A.Ö.); SR4 (H.G.H.Ç.) | 23 | 20 | — |
The Background of Radiologists
3.3. Question Development and Validation
The study utilized a total of 210 questions based on key knowledge from TSR-2018 MCASG, comprising 105 OEQs and 105 CBQs from different sections (Breast, Abdomen and Pelvis, Musculoskeletal, Brain, Cardiothoracic, Spinal, and Head and Neck). There were 15 questions for each section in both question formats (Table 2). These questions were created through a three-step workflow.
| Question type | Question number per section (n) | Sections | Total number of questions (n) | Purpose of question type |
|---|---|---|---|---|
| OEQs | 15 | 7 | 105 | Assess factual knowledge of MRI acquisition standards |
| CBQs | 15 | 7 | 105 | Identify single, key MRI sequence for a given clinical scenario |
The Question Set of the Study
A. Stage 1 (item generation): The JR3 drafted 30 OEQs and 30 CBQs for each of the seven sections (n = 420), drawing exclusively on TSR-2018 MCASG.
B. Stage 2 [expert consensus (modified Delphi)]: The SR3 and SR4 independently evaluated and rated every question for clinical relevance and clarity. Questions with a Content-Validity Index < 0.80 were revised and re-rated; after two rounds, ≥ 90% inter-rater agreement was achieved. The process yielded the final 15 OEQs and 15 CBQs per subspecialty (n = 210).
C. Stage 3 (pilot clarity testing): The consensus set was trialed with two different radiology residents (in the second and last year of their residency) who were not study participants. Feedback resulted in minor wording changes; no questions were discarded.
Because the questions are rule-based with objectively correct answers anchored in TSR-2018 MCASG, formal difficulty indexing or factor analysis was not pursued. The OEQs were clearly formulated, each addressing a single, specific concept to effectively evaluate knowledge on MRI acquisition standards such as technical parameters, protocol considerations, imaging sequences, and patient preparation and positioning. Correspondingly, the CBQs were designed to identify the most appropriate MRI sequence for particular clinical conditions, typically providing one definitive answer; however, in certain cases, two sequences were deemed equally appropriate, with either considered correct. Supplementary Materials list OEQs and CBQs with their datasets.
3.4. Prompting and Model Input Procedures
We used the following prompt for OEQs: "Act like a radiology professor with twenty-five years of experience in radiology. You perform MRI acquisitions according to 'TRD MRG ve BT İnceleme Standartları 2018.' I will ask you open-ended questions about MRI acquisition standards. Answer me with sentences that you think are the most appropriate answer to the question. Each question is an independent task. Do not assume relation to previous or following questions".
For CBQs, the following input prompt was used: "Act like a radiology professor with twenty-five years of experience in radiology. You perform MRI acquisitions according to 'TRD MRG ve BT İnceleme Standartları 2018.' I will provide you with clinical information about my patients, including their diagnoses and special conditions, and clinical pre-diagnosis that I would like to reach the most likely diagnosis and differentiate them from differentials. We will plan magnetic resonance imaging (MRI) for these patients. Indicate the key MRI sequence that must be included in the acquisition protocol for these patients and is absolutely necessary for me to make the indicated diagnosis. Give me only one most important, a must-have key sequence. Each question is an independent task. Do not assume relation to previous or following questions".
These prompts followed a structured, zero-shot format without any iterative refinement during the study. They employed role-based contextualization to emulate the reasoning process of a SR, with the intent to enhance clinical relevance and promote detailed differential generation. To avoid potential bias from variable prompt construction across whole sessions, a single prompt format was used. To eliminate carry-over context, each model was tested in a fresh session per format with no prior conversation history or memory activated. Context-resetting measures were taken where applicable.
All models were used with default hyperparameter settings as provided in their publicly available web interfaces as of January 2025. No fine-tuning or API-level parameter manipulation was applied. We used the web-based front ends to ensure evaluation under standard user conditions. These standardization procedures were applied across all eight LLMs to control for prompt variability and ensure that observed differences in performance were attributable to model behavior rather than prompt structure or system configuration. These prompts were administered in January 2025 across eight different models: Anthropic’s Claude 3 Opus and Claude 3.5 Sonnet (https://claude.ai.com), OpenAI’s ChatGPT-4o with canvas, ChatGPT-4o, and ChatGPT-o1 (https://chat.openai.com), Google Gemini 1.5 Pro (https://aistudio.google.com), Mistral Large 2 (https://mistral.ai), and Meta Llama 3.1 405B (https://metaai.com).
The LLMs were not subjected to any additional pre-training or fine-tuning by the authors before the study, and no specific information or criteria that might influence the research objectives or outcomes were provided (Figures 2A and B).
3.5. Performance Evaluation
The responses to OEQs were independently evaluated by JR3 and SR3 using a 4-point Likert scale:
A. 1 point: Completely incorrect.
B. 2 points: Mostly incorrect.
C. 3 points: Mostly correct.
D. 4 points: Completely correct.
To reduce potential bias, all responses for each question were exported as plain-text files and anonymized using alphanumeric codes by an independent radiology resident not involved in scoring and not participating in the study. This ensured that evaluators were blinded to the identity of the respondent (LLMs and radiologists) and scored the responses without knowledge of the source.
For the evaluation of OEQs, SR4 authored concise model answers encapsulating the core technical or protocol concept mandated by the guideline. These reference answers, plus a 4-point Likert rubric, were supplied to the two evaluators (JR3 and SR3). Ratings were assigned independently using the reference answer key as the benchmark. The JR3 and SR3 reviewed the responses independently. After that, the discrepancies were resolved via in-person discussion. The pre-consensus interobserver agreement was substantial, with a weighted Cohen’s κ of 0.864 (95% CI, 0.821 - 0.902), indicating high scoring consistency.
For the evaluation of CBQs, SR4 derived a single key (indispensable) MRI sequence for each question strictly from TSR-2018 MCASG. Where the guideline allowed two equally critical sequences, both were entered into the answer key. This answer key constituted the gold standard. CBQ responses were independently scored by JR3 and SR3 using binary scoring (correct = 1, incorrect = 0).
3.6. Statistical Analysis
Descriptive statistics were represented using percentages. Subsequently, Tamhane’s T2 procedure was employed for post hoc multiple comparisons to delineate specific intergroup differences. McNemar’s test was used to compare the proportion of correct responses between different questions. The Wilcoxon test was used to compare Likert scores. For paired comparisons of Likert scores and accuracy between radiologists and LLMs, a Bonferroni correction was applied to adjust for multiple comparisons. Specifically, with 120 pairwise comparisons across eight LLMs and eight radiologists, statistical significance was defined as P < 0.0004. All statistical analyses were performed using SPSS version 26.0 (IBM Corp. Released 2019. IBM SPSS Statistics for Windows, Version 26.0. Armonk, NY: IBM Corp).
4. Results
4.1. Open-ended Question Performance
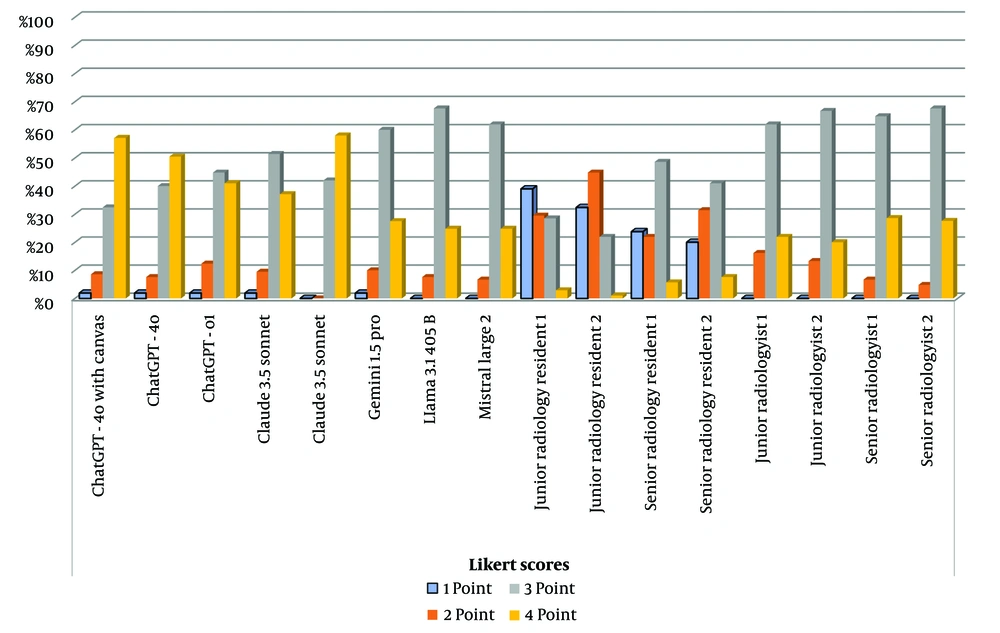
Claude 3.5 Sonnet achieved the highest performance of 3.51 ± 0.54 (median: 4), followed by ChatGPT-4o with canvas at 3.45 ± 0.73 (median: 3) and ChatGPT-4o at 3.39 ± 0.71 (median: 3). ChatGPT-o1 and Claude 3 Opus demonstrated comparable performance, with scores of 3.25 ± 0.74 (median: 3) and 3.24 ± 0.70 (median: 3), respectively. Gemini 1.5 Pro recorded a mean score of 3.13 ± 0.67 (median: 3), while Llama 3.1 405B and Mistral Large 2 achieved mean scores of 3.17 ± 0.55 (median: 3) and 3.25 ± 0.57 (median: 3), respectively. The JRR1 and JRR2 recorded means of 1.95 ± 0.89 (median: 2) and 1.91 ± 0.76 (median: 2), respectively. The SRR1 and SRR2 demonstrated slightly higher performances of 2.36 ± 0.90 (median: 3) and 2.36 ± 0.89 (median: 3), respectively. The JR1 and JR2 achieved a mean of 3.06 ± 0.62 (median: 3) and 3.07 ± 0.58 (median: 3), respectively. The SR1 recorded a mean of 3.22 ± 0.55 (median: 3), while SR2 achieved the highest performance among radiologists with 3.23 ± 0.52 (median: 3) (Figure 3 and Table 3).
| Variables | Mean Likert score (point) | 95% CI lower (point) | 95% CI upper (point) |
|---|---|---|---|
| Claude 3.5 Sonnet | 3.51 | 3.39 | 3.63 |
| ChatGPT-4o with canvas | 3.45 | 3.28 | 3.62 |
| ChatGPT-4o | 3.39 | 3.22 | 3.56 |
| ChatGPT-o1 | 3.25 | 3.08 | 3.42 |
| Claude 3 Opus | 3.24 | 3.08 | 3.4 |
| Mistral Large 2 | 3.25 | 3.12 | 3.38 |
| Llama 3.1 405B | 3.17 | 3.04 | 3.3 |
| Gemini 1.5 Pro | 3.13 | 2.99 | 3.27 |
| JRR1 | 1.95 | 1.73 | 2.17 |
| JRR2 | 1.91 | 1.65 | 2.17 |
| SRR1 | 2.36 | 2.13 | 2.59 |
| SRR2 | 2.36 | 2.13 | 2.59 |
| JR1 | 3.06 | 2.92 | 3.2 |
| JR2 | 3.07 | 2.93 | 3.21 |
| SR1 | 3.22 | 3.09 | 3.35 |
| SR2 | 3.23 | 3.1 | 3.36 |
Descriptive Statistics for Open-ended Questions
There was no significant difference in LLM performance across the sections (e.g., brain, abdomen, MSK) at OEQs. The overall effect size for OEQ performance was large (η2 = 0.48), indicating substantial variability between groups. The pairwise effect size between Claude 3.5 Sonnet and Gemini 1.5 Pro was large (Cohen’s d = 0.79, 95% CI: 0.52 - 1.06). Similarly, the effect size between Claude 3.5 Sonnet and Mistral Large 2 was Cohen’s d = 0.57 (95% CI: 0.30 - 0.83). Claude 3.5 Sonnet outperformed Gemini 1.5 Pro (P = 0.0001), Mistral Large 2 (P = 0.0002), Llama 3.1 405B (P = 0.0001), and radiologists (JRR1, JRR2, SRR1, SRR2, JR1, JR2, SR1, and SR2) (P < 0.0004). There was no significant difference between the performance of other LLMs (P > 0.0004). Radiology residents underperformed significantly when compared to LLMs and other radiologists (P < 0.0004). The comparison of the performance of LLMs and radiologists at OEQs is shown in Table 4.
| Variables | Claude 3 Opus | Claude 3.5 Sonnet | ChatGPT-4o | Mistral Large 2 | ChatGPT-4o with canvas | Gemini 1.5 Pro | ChatGPT-o1 | Llama 3.1 405B | JRR-1 | JRR-2 | SRR-1 | SRR-2 | JR-1 | JR-2 | SR-1 | SR-2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Claude 3 Opus | - | 0.0020 | 0.0640 | 0.9070 | 0.0100 | 0.2470 | 0.9110 | 0.3400 | 0.0002 | 0.0002 | 0.0002 | 0.0002 | 0.0420 | 0.0400 | 0.8390 | 0.7590 |
| Claude 3.5 Sonnet | 0.0020 | - | 0.1580 | 0.0002 | 0.4550 | 0.0001 | 0.0040 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0002 | 0.0002 | 0.0002 |
| ChatGPT-4o | 0.0640 | 0.1580 | - | 0.0910 | 0.3170 | 0.0050 | 0.0800 | 0.3200 | 0.0001 | 0.0001 | 0.0003 | 0.0003 | 0.0010 | 0.0010 | 0.4900 | 0.5200 |
| Mistral Large 2 | 0.9070 | 0.0002 | 0.0910 | - | 0.0230 | 0.1350 | 0.9590 | 0.2580 | 0.0001 | 0.0001 | 0.0002 | 0.0003 | 0.0340 | 0.0380 | 0.7230 | 0.7020 |
| ChatGPT-4o with canvas | 0.0100 | 0.4550 | 0.3170 | 0.0230 | - | 0.0010 | 0.0050 | 0.0010 | 0.0001 | 0.0001 | 0.0002 | 0.0002 | 0.0010 | 0.0010 | 0.0120 | 0.0110 |
| Gemini 1.5 Pro | 0.2470 | 0.0001 | 0.0050 | 0.1350 | 0.0010 | - | 0.3390 | 0.6150 | 0.0003 | 0.0002 | 0.0002 | 0.0001 | 0.3800 | 0.3110 | 0.2700 | 0.2340 |
| ChatGPT-o1 | 0.9110 | 0.0040 | 0.0800 | 0.9590 | 0.0050 | 0.3390 | - | 0.3790 | 0.0001 | 0.0001 | 0.0002 | 0.0003 | 0.0350 | 0.0310 | 0.7920 | 0.7000 |
| Llama 3.1 405B | 0.3400 | 0.0001 | 0.3200 | 0.2580 | 0.0010 | 0.6150 | 0.3790 | - | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.1700 | 0.1230 | 0.5400 | 0.5080 |
| JRR-1 | 0.0002 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0003 | 0.0001 | 0.0001 | - | 0.7510 | 0.0002 | 0.0003 | 0.0002 | 0.0002 | 0.0002 | 0.0002 |
| JRR-2 | 0.0002 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0002 | 0.0001 | 0.0001 | 0.7510 | - | 0.0002 | 0.0002 | 0.0001 | 0.0002 | 0.0001 | 0.0001 |
| SRR-1 | 0.0002 | 0.0001 | 0.0003 | 0.0002 | 0.0002 | 0.0002 | 0.0003 | 0.0001 | 0.0003 | 0.0002 | - | 0.8210 | 0.0001 | 0.0002 | 0.0002 | 0.0002 |
| SRR-2 | 0.0002 | 0.0001 | 0.0003 | 0.0003 | 0.0002 | 0.0001 | 0.0003 | 0.0001 | 0.0003 | 0.0002 | 0.8210 | - | 0.0002 | 0.0002 | 0.0001 | 0.0001 |
| JR-1 | 0.0420 | 0.0002 | 0.0010 | 0.0340 | 0.0010 | 0.3800 | 0.0350 | 0.1700 | 0.0002 | 0.0001 | 0.0001 | 0.0002 | - | 0.8600 | 0.0002 | 0.0002 |
| JR-2 | 0.0400 | 0.0002 | 0.0010 | 0.0380 | 0.0010 | 0.3110 | 0.0310 | 0.1230 | 0.0002 | 0.0002 | 0.0002 | 0.0002 | 0.8600 | - | 0.0003 | 0.0002 |
| SR-1 | 0.8390 | 0.0002 | 0.4900 | 0.7230 | 0.0120 | 0.2700 | 0.7920 | 0.5400 | 0.0002 | 0.0001 | 0.0002 | 0.0001 | 0.0002 | 0.0003 | - | 0.6400 |
| SR-2 | 0.7590 | 0.0002 | 0.5200 | 0.7020 | 0.0110 | 0.2340 | 0.7000 | 0.5080 | 0.0002 | 0.0001 | 0.0002 | 0.0001 | 0.0002 | 0.0002 | 0.6400 | - |
Comparison of the Performance of Large Language Models and Radiologists at Open-ended Questions a
4.2. Case-based Question Accuracy
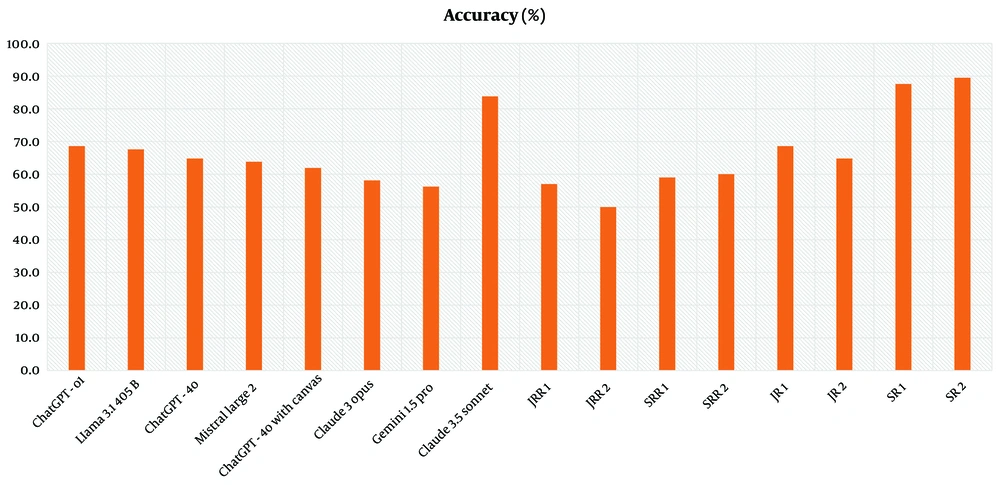
The LLMs revealed the following accuracy rates (the proportion of correctly selected key MRI sequences by the models and radiologists in response to clinical case-based scenarios) at CBQs: ChatGPT-4o with canvas (61.9%), ChatGPT-4o (64.8%), ChatGPT-o1 (68.6%), Claude 3 Opus (58.1%), Claude 3.5 Sonnet (83.8%), Gemini 1.5 Pro (56.2%), Llama 3.1 405B (67.6%), and Mistral Large 2 (63.8%). The JRRs achieved 57% and 50%, while SRRs scored 59% and 60%. JR1 and JR2 achieved accuracy rates of 68.6% and 64.8%, respectively, while SR1 recorded 87.6%, and SR2 demonstrated accuracy at 89.5% (Figure 4 and Table 5).
| Variables | CBQ accuracy (%) | 95% CI lower (%) | 95% CI upper (%) |
|---|---|---|---|
| Claude 3.5 Sonnet | 84 | 78 | 90 |
| SR2 | 90 | 84 | 96 |
| SR1 | 88 | 82 | 94 |
| ChatGPT-o1 | 69 | 61 | 77 |
| Llama 3.1 405B | 68 | 60 | 76 |
| JR1 | 69 | 61 | 77 |
| ChatGPT-4o | 65 | 57 | 73 |
| JR2 | 65 | 57 | 73 |
| Mistral Large 2 | 64 | 56 | 72 |
| ChatGPT-4o with canvas | 62 | 54 | 70 |
| SRR2 | 60 | 52 | 68 |
| SRR1 | 59 | 51 | 67 |
| Claude 3 Opus | 58 | 50 | 66 |
| JRR1 | 57 | 49 | 65 |
| Gemini 1.5 Pro | 56 | 48 | 64 |
| JRR2 | 50 | 42 | 58 |
Descriptive Statistics for Case-based Questions
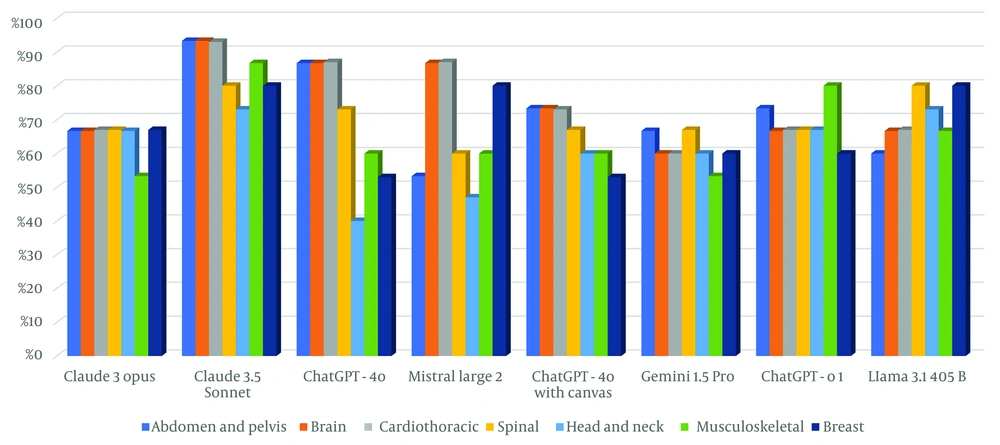
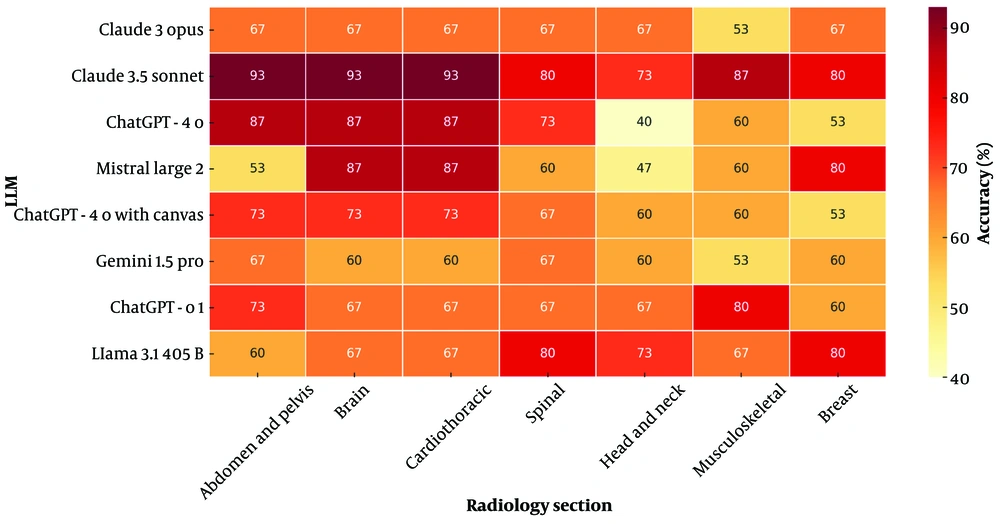
Claude 3.5 Sonnet demonstrated superior performance among LLMs across all sections at CBQs. It consistently achieved ≥ 73% accuracy across all subspecialties and excelled in the “Abdomen and Pelvis”, “Brain”, “Cardiothoracic”, and “Spinal” sections with 93.3% accuracy (Figures 5 and 6). The performance of LLMs at CBQs and OEQs according to sections is shown in Table 6 (P > 0.05).
| Variables | Abdomen and pelvis | Brain | Cardiothoracic | Spinal | Head and neck | Musculoskeletal | Breast | P |
|---|---|---|---|---|---|---|---|---|
| Claude 3 Opus (CBQ) | 0.239 X2 | |||||||
| False | 5 (33.3) | 5 (33.3) | 5 (33.3) | 5 (33.3) | 5 (33.3) | 7 (46.7) | 5 (33.3) | |
| True | 10 (66.7) | 10 (66.7) | 10 (66.7) | 10 (66.7) | 10 (66.7) | 8 (53.3) | 10 (66.7) | |
| Claude 3 Opus Likert Score (OEQ) | 0.592 K | |||||||
| Mean ± SD | 3.07± 0.594 | 3.47± 0.516 | 3.07 ± 0.799 | 3.33 ± 0.617 | 3.40 ± 0.632 | 3.13 ± 0.990 | 3.20 ± 0.676 | |
| Median | 3 | 3 | 3 | 3 | 3 | 3 | 3 | |
| Claude 3.5 Sonnet (CBQ) | 0.710 X2 | |||||||
| False | 1 (6.7) | 1 (6.7) | 1 (6.7) | 3 (20.0) | 4 (26.7) | 2 (13.3) | 3 (20.0) | |
| True | 14 (93.3) | 14 (93.3) | 14 (93.3) | 12 (80.0) | 11 (73.3) | 13 (86.7) | 12 (80.0) | |
| Claude-3.5 Sonnet Likert Score (OEQ) | 0.670 K | |||||||
| Mean ± SD | 3.60 ± 0.507 | 3.60± 0.507 | 3.60 ± 0.507 | 3.67 ± 0.488 | 3.67 ± 0.488 | 3.47 ± 0.516 | 3.47 ± 0.516 | |
| Median | 4 | 4 | 4 | 4 | 4 | 3 | 3 | |
| ChatGPT-4o (CBQ) | 0.050 X2 | |||||||
| False | 2 (13.3) | 2 (13.3) | 2 (13.3) | 4 (26.7) | 9 (60.0) | 6 (40.0) | 7 (46.7) | |
| True | 13 (86.7) | 13 (86.7) | 13 (86.7) | 11 (73.3) | 6 (40.0) | 9 (60.0) | 8 (53.3) | |
| ChatGPT-4o Likert Score (OEQ) | 0.717 K | |||||||
| Mean ± SD | 3.47 ± 0.516 | 3.47± 0.516 | 3.53 ± 0.640 | 3.33 ± 0.724 | 3.47 ± 0.640 | 3.20 ± 1.014 | 3.13 ± 0.834 | |
| Median | 3 | 3 | 4 | 4 | 4 | 3 | 3 | |
| Mistral Large 2 (CBQ) | 0.210 X2 | |||||||
| False | 7 (46.7) | 2 (13.3) | 2 (13.3) | 6 (40.0) | 8 (53.3) | 6 (40.0) | 3 (20.0) | |
| True | 8 (53.3) | 13 (86.7) | 13 (86.7) | 9 (60.0) | 7 (46.7) | 9 (60.0) | 12 (80.0) | |
| Mistral Large 2 Likert Score (OEQ) | 0.423 K | |||||||
| Mean ± SD | 3.20 ± 0.561 | 3.20± 0.561 | 3.33 ± 0.617 | 3.13 ± 0.516 | 3.33 ± 0.617 | 3.47 ± 0.640 | 3.07 ± 0.458 | |
| Median | 3 | 3 | 3 | 3 | 3 | 4 | 3 | |
| ChatGPT-4o with canvas (CBQ) | 0.709 X2 | |||||||
| False | 4 (26.7) | 4 (26.7) | 4 (26.7) | 5 (33.3) | 6 (40.0) | 6 (40.0) | 7 (46.7) | |
| True | 11 (73.3) | 11 (73.3) | 11 (73.3) | 10 (66.7) | 9 (60.0) | 9 (60.0) | 8 (53.3) | |
| ChatGPT-4o with canvas Likert Score (OEQ) | 0.128 K | |||||||
| Mean ± SD | 3.20 ± 0.775 | 3.53± 0.640 | 3.80 ± 0.414 | 3.60 ± 0.737 | 3.53 ± 0.640 | 3.27 ± 1.033 | 3.20 ± 0.676 | |
| Median | 3 | 3 | 4 | 3 | 4 | 4 | 3 | |
| Gemini 1.5 Pro (CBQ) | 0.648 X2 | |||||||
| False | 5 (33.3) | 6 (40.0) | 6 (40.0) | 5 (33.3) | 6 (40.0) | 7 (46.7) | 6 (40.0) | |
| True | 10 (66.7) | 9 (60.0) | 9 (60.0) | 10 (66.7) | 9 (60.0) | 8 (53.3) | 9 (60.0) | |
| Gemini 1.5 Pro Likert Score (OEQ) | 0.761 K | |||||||
| Mean ± SD | 3.20 ± 0.561 | 2.93± 0.799 | 3.07 ± 0.594 | 3.27 ± 0.594 | 3.33 ± 0.617 | 3.07 ± 0.799 | 3.07 ± 0.704 | |
| Median | 3 | 3 | 3 | 3 | 3 | 3 | 3 | |
| ChatGPT-o1 (CBQ) | 0.948 X2 | |||||||
| False | 4 (26.7) | 5 (33.3) | 5 (33.3) | 5 (33.3) | 5 (33.3) | 3 (20.0) | 6 (40.0) | |
| True | 11 (73.3) | 10 (66.7) | 10 (66.7) | 10 (66.7) | 10 (66.7) | 12 (80.0) | 9 (60.0) | |
| ChatGPT-o1 Likert Score (OEQ) | 0.912 K | |||||||
| Mean ± SD | 3.27 ± 0.799 | 3.47± 0.516 | 3.20 ± 0.775 | 3.27 ± 0.594 | 3.27 ± 0.704 | 3.20 ± 1. 014 | 3.07 ± 0.799 | |
| Median | 3 | 3 | 3 | 3 | 3 | 3 | 3 | |
| Llama 3.1 405B (CBQ) | 0.459 X2 | |||||||
| False | 6 (40.0) | 5 (33.3) | 5 (33.3) | 3 (20.0) | 4 (26.7) | 5 (33.3) | 3 (20.0) | |
| True | 9 (60.0) | 10 (66.7) | 10 (66.7) | 12 (80.0) | 11 (73.3) | 10 (66.7) | 12 (80.0) | |
| Llama 3.1 405B Likert Score (OEQ) | 0.752 K | |||||||
| Mean ± SD | 3.20 ± 0.561 | 3.27± 0.458 | 3.13 ± 0.516 | 3.07 ± 0.594 | 3.00 ± 0.535 | 3.27 ± 0.594 | 3.27 ± 0.594 | |
| Median | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
The Performances of Large Language Models Across Sections a
The overall effect size for CBQ accuracy was large (η2 = 0.52), suggesting substantial between-group variability. The pairwise effect size between Claude 3.5 Sonnet and Gemini 1.5 Pro was large (Cohen’s d = 1.41, 95% CI: 1.07 - 1.75). Between Claude 3.5 Sonnet and Mistral Large 2, Cohen’s d was 1.34 (95% CI: 1.01 - 1.67). Claude 3.5 Sonnet outperformed Claude 3 Opus (P = 0.0001), ChatGPT-4o with canvas (P = 0.0001), Gemini 1.5 Pro (P = 0.0002), Mistral Large 2 (P = 0.0003), Llama 3.1 405B (P = 0.0001), and all radiologists (P < 0.0004).
There was no significant difference between the performance of other LLMs (P > 0.0004). Radiology residents underperformed significantly when compared to SRs (P < 0.0004). The comparison of the performance of all LLMs and radiologists at CBQs is shown in Table 7.
| Variables | Claude 3 Opus | Claude 3.5 Sonnet | ChatGPT-4o | Mistral Large 2 | ChatGPT-4o with canvas | Gemini 1.5 Pro | ChatGPT-o1 | Llama 3.1405B | JRR-1 | JRR-2 | SRR-1 | SRR-2 | JR-1 | JR-2 | SR-1 | SR-2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Claude 3 Opus | - | 0.0001 | 0.0907 | 0.1650 | 0.1700 | 0.7280 | 0.0300 | 0.0270 | 0.5510 | 0.6710 | 0.2810 | 0.4100 | 0.0300 | 0.2810 | 0.0002 | 0.0002 |
| Claude 3.5 Sonnet | 0.0001 | - | 0.0020 | 0.0003 | 0.0001 | 0.0002 | 0.0150 | 0.0120 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0002 | 0.0002 |
| ChatGPT-4o | 0.0970 | 0.0020 | - | 1 | 0.5130 | 0.1760 | 0.5960 | 0.6350 | 0.2650 | 0.0500 | 0.7600 | 0.5960 | 0.6430 | 0.7750 | 0.0001 | 0.0001 |
| Mistral Large 2 | 0.1650 | 0.0003 | 1 | - | 0.8620 | 0.2300 | 0.4860 | 0.4050 | 0.4610 | 0.0610 | 1 | 0.6880 | 0.4880 | 0.8880 | 0.0001 | 0.0001 |
| ChatGPT-4o with canvas | 0.1700 | 0.0001 | 0.5130 | 0.8620 | - | 0.3550 | 0.1940 | 0.2620 | 0.1510 | 0.0700 | 1 | 0.7850 | 0.2500 | 1 | 0.0002 | 0.0002 |
| Gemini 1.5 Pro | 0.7280 | 0.0002 | 0.1760 | 0.2300 | 0.3550 | - | 0.0310 | 0.0430 | 0.8770 | 0.3710 | 0.4960 | 0.6880 | 0.0990 | 0.4700 | 0.0001 | 0.0001 |
| ChatGPT-o1 | 0.0300 | 0.0150 | 0.5960 | 0.4860 | 0.1940 | 0.0310 | - | 1 | 0.1090 | 0.0070 | 0.3600 | 0.2220 | 1 | 0.3600 | 0.0030 | 0.0010 |
| Llama 3.1 405B | 0.0270 | 0.0120 | 0.6350 | 0.4050 | 0.2620 | 0.0430 | 1 | - | 0.1530 | 0.0030 | 0.3710 | 0.2720 | 1 | 0.4010 | 0.0010 | 0.0001 |
| JRR-1 | 0.5510 | 0.0001 | 0.2650 | 0.4610 | 0.1510 | 0.8770 | 0.1090 | 0.1530 | - | 0.3130 | 0.6260 | 0.8900 | 0.1090 | 0.6580 | 0.0001 | 0.0002 |
| JRR-2 | 0.6710 | 0.0001 | 0.0500 | 0.0610 | 0.0700 | 0.3710 | 0.0070 | 0.0030 | 0.3130 | - | 0.0800 | 0.1360 | 0.0140 | 0.1060 | 0.0002 | 0.0002 |
| SRR-1 | 0.2810 | 0.0001 | 0.7600 | 1 | 1 | 0.4960 | 0.3600 | 0.3710 | 0.6260 | 0.0800 | - | 0.8900 | 0.3370 | 1 | 0.0002 | 0.0002 |
| SRR-2 | 0.4100 | 0.0001 | 0.5960 | 0.6880 | 0.7850 | 0.6880 | 0.2220 | 0.2720 | 0.8900 | 0.1360 | 0.8900 | - | 0.2720 | 0.8740 | 0.0002 | 0.0002 |
| JR-1 | 0.0300 | 0.0001 | 0.6430 | 0.4880 | 0.2500 | 0.0990 | 1 | 1 | 0.1090 | 0.0140 | 0.3370 | 0.2720 | - | 0.4420 | 0.0002 | 0.0010 |
| JR-2 | 0.2810 | 0.0001 | 0.7750 | 0.8880 | 1 | 0.4700 | 0.3600 | 0.4010 | 0.6580 | 0.1060 | 1 | 0.8740 | 0.4420 | - | 0.0800 | 0.1360 |
| SR-1 | 0.0002 | 0.5410 | 0.0001 | 0.0001 | 0.0002 | 0.0001 | 0.0030 | 0.0010 | 0.0001 | 0.0002 | 0.0002 | 0.0002 | 0.0002 | 0.0800 | - | 0.8900 |
| SR-2 | 0.0002 | 0.0002 | 0.0001 | 0.0001 | 0.0002 | 0.0001 | 0.0010 | 0.0001 | 0.0002 | 0.0002 | 0.0002 | 0.0002 | 0.0010 | 0.1360 | 0.8900 | - |
Comparison of the Performance of Large Language Models and Radiologists at Case Based Questions a
5. Discussion
5.1. Summary of Key Findings
The most noteworthy finding of this study is the superior performance of the Claude 3.5 Sonnet model in adhering to MRI acquisition standards across nearly all sections of radiology. This model outperformed SRs in selecting the most important sequence that must be included in the MRI acquisition protocol. Claude 3.5 Sonnet shows impressive performance with 83.8% accuracy on CBQs and scored high with an average of 3.51 (± 0.54) and a median of 4 on OEQs. This suggests that it could be a pioneer model in this field.
Another important result of our study is that all LLMs had comparable proficiency in both OEQs and CBQs to JRs. This shows that recent LLMs show strong potential as helpful tools in MRI acquisition and sequence selection. In addition to this, our study uniquely compares the performance of different LLMs on MRI acquisition standards knowledge and key sequence selection, and it shows the valuable potential of these models by comparing them to radiologists across different experience levels.
The performance variations among LLMs can be primarily attributed to their distinct architectural frameworks and training methodologies. A notable observation was that internet-enabled models like Gemini 1.5 Pro demonstrated lower accuracy compared to their offline counterparts, likely due to their tendency to retrieve information from non-peer-reviewed sources. In contrast, ChatGPT models and Claude models operate on proprietary, curated datasets specifically designed for medical and scientific applications.
When the performances of LLMs included were compared according to sections, there was no significant difference in both OEQs and CBQs according to the sections (P > 0.05). This result shows that current LLMs have a similar level of competence and knowledge not only in certain sections but also in almost all sections that we frequently encounter in radiology practice.
5.2. Comparison with Existing Literature
Prior studies have largely addressed the radiological knowledge of LLMs and their support for post-imaging tasks such as lesion detection, image segmentation, and automated reporting (5, 15-18). Bhayana et al. evaluated ChatGPT's performance on 150 MCQs designed to match the style and rigor of Canadian Royal College and American Board of Radiology examinations, demonstrating that the model correctly answered nearly 70% of all questions. Notably, ChatGPT excelled in lower-order cognitive tasks — those requiring recall or understanding — achieving an 84% success rate, and also performed strongly (89%) on higher-order clinical management questions (16).
In another study, Ariyaratne et al. assessed ChatGPT’s suitability for radiology board-style assessments; GPT-4 was tested on question banks mirroring parts 1 and 2A of the Fellowship of the Royal College of Radiologists (FRCR) examination. Although GPT-4 answered nearly 75% of part 1 true/false questions correctly — a score marginally below the established passing mark — its performance on 2A single best answer questions was notably stronger, achieving a 74.2% accuracy rate and comfortably surpassing the passing threshold of 63.3% (19).
Beyond text-based knowledge, Horiuchi et al. found that ChatGPT 4 performed comparably well to a radiology assistant, although not as well as a board-certified radiologist, with an accuracy of 43% on 106 “Test Yourself” cases from Skeletal Radiology (20).
5.3. Implications for Radiology Practice
The contribution that LLMs can make to radiology goes beyond radiological image assessment. Mese et al. emphasized that by generating customized assessments, translating complex materials, and summarizing large volumes of data, ChatGPT can serve as a flexible tutor around the clock. Using these capabilities of ChatGPT in radiology education can provide a more holistic, student-centered environment by advancing critical thinking and professional skill development without eliminating the essential role of traditional teaching methods (21).
Lyu et al. translated both computed tomography and MRI reports into different languages with ChatGPT, and radiologists evaluated the reports translated by ChatGPT on a 5-point system for accuracy and adequacy. In this study, ChatGPT-4 achieved a score of 4.27, and it was stated that it has great potential in this regard (22).
In another study, Sievert et al. performed risk stratification of thyroid nodules according to the Thyroid Imaging Reporting and Data System (TI-RADS) with ChatGPT on anonymized radiology reports and stated that it offers an important future to guide clinicians in this regard (23). Zaki et al. demonstrated that LLMs are highly effective in determining the most appropriate imaging modality (12).
Beyond previous studies, this study broadens the scope of LLMs' role to include strategic decision-making before an acquisition is even performed by demonstrating the great performance of LLMs in patient-specific key sequence selection (24-27). The MRI acquisition optimization and sequence selection have critical importance as fundamental aspects for radiologists. Our study contributes valuable insights and a different approach by evaluating their proficiency in MRI acquisition standards and key sequence selection.
5.4. Study Limitations
This study has several limitations. First, we used one standardized prompt for each question. We did not test how different prompts might affect LLM responses, as this was beyond our study's aim. Since the input prompt significantly influences LLM performance, using optimized prompts could potentially yield better results. Further studies are needed to understand how different prompts affect LLM responses in this subject.
Second, both question formats employed in this study were intentionally simplified for controlled benchmarking. The OEQs required concise, single-point answers rather than the stepwise narrative reasoning that characterizes day-to-day consultation with clinical teams. Likewise, the CBQs focused on selecting a single, highest-yield MRI sequence per vignette, thereby omitting the diagnostic uncertainty and multi-sequence protocols often necessary in routine practice. Consequently, our design does not fully capture the complexity, ambiguity, and iterative decision-making present in real-world radiology. Future work should therefore validate LLM performance within authentic clinical workflows — preferably through prospective studies or large retrospective imaging datasets that incorporate the full spectrum of patient presentations and imaging requirements.
Also, while the question set was developed using expert consensus and piloted for clarity, formal psychometric validation — such as item difficulty analysis, discrimination indices, or test - retest reliability — was not performed. This may limit the generalizability of item-level findings. Future studies should incorporate comprehensive psychometric evaluation across broader learner cohorts to further establish the reliability and validity of the assessment tool.
Third, MRI acquisition standards in different centers and countries could show differences with minor variations. In this study, we performed our evaluations according to TSR-2018 MCASG, but the performance of LLMs may vary with different MRI acquisition guidelines. Further studies including different MRI acquisition guidelines are needed to show that the performance of LLMs in our study is generalizable to other guidelines. Finally, LLM development is an ongoing process, with models continuously improving through new knowledge and reinforcement learning. Therefore, our results reflect the models' capabilities only at the time of this study. Future versions of these models may show improved performance in this field.
5.5. Directions for Future Research
Further multicenter studies are warranted to corroborate our findings under authentic clinical conditions, wherein diagnostic uncertainty, patient heterogeneity, and complex multi-sequence protocols more closely mirror routine radiologic workflows. Such studies should incorporate large, longitudinal imaging datasets governed by diverse acquisition guidelines — extending beyond TSR-2018 MCASG — to determine the external validity of current LLMs. Rigorous experimentation with advanced prompt-engineering strategies, adaptive conversational frameworks, and iterative human-in-the-loop feedback will be essential to optimize model performance and mitigate context-specific biases. Finally, longitudinal evaluations charting successive model iterations, together with implementation studies that assess integration into radiologist reporting, protocol planning, and trainee education, will be critical for delineating the true clinical utility and safety profile of LLM-enabled decision support in MRI practice.
In conclusion, particularly Claude 3.5 Sonnet, performed robustly in our simulated benchmark, accurately selecting key MRI sequences and adhering to guideline-based acquisition standards across multiple subspecialties. These results highlight the potential of advanced LLMs as decision-support aids during protocol planning. Future studies incorporating real clinical scenarios and authentic patient data are critical for realizing this great potential of LLMs and evaluating their prospective transformative role in radiologic practice.