1. Introduction
Multiple myeloma (MM) is characterized by abnormal proliferation of plasma cells, usually originating in the bone marrow. The most common manifestation is bone involvement with radiologic skeletal findings of diffuse osteopenia or osteolytic bone lesions. A total of 7 - 18% of MM cases are associated with extramedullary (EM) involvement at the time of diagnosis (1). EM plasmacytoma in MM may occur during the course of disease or at relapse. It can arise from any tissue, and its presence has been associated with more aggressive disease (2). One of the diagnostic criteria of MM is the detection of monoclonal gammopathy in serum and/or urine electrophoresis. When it is not present, the condition is known as non-secretory multiple myeloma, making the diagnosis more difficult. This subtype of MM represents approximately 1 - 4% of all MM cases (3).
2. Case Presentation
A 31-year-old lady presented with a three-week history of right shoulder pain associated with lethargy, palpitation and shortness of breath. The right shoulder pain was not preceded by trauma. Her past medical history was unremarkable. Physical examination revealed intense tenderness around the right shoulder. However, there was no limitation of movement. She was pale, with a blood pressure of 110/50 mmHg and heart rate of 98/min. Multiple firm, mobile, non-tender breast lumps were palpated bilaterally. However, no axillary lymphadenopathy was detected. There was bilateral proptosis more dominant on the right side. The rest of the physical examination was unremarkable. Blood investigations showed normochromic normocytic anemia: the hemoglobin level measured 6.5 g/dL. The rest of the blood investigations were unremarkable with normal serum calcium level (2.49 mmol/L).
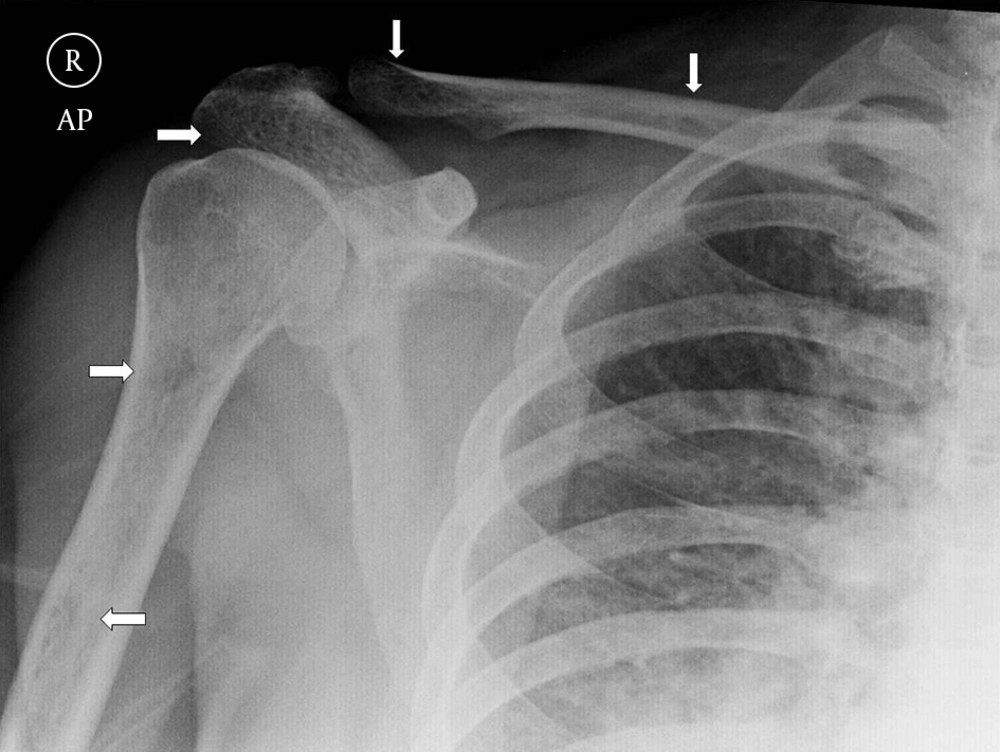
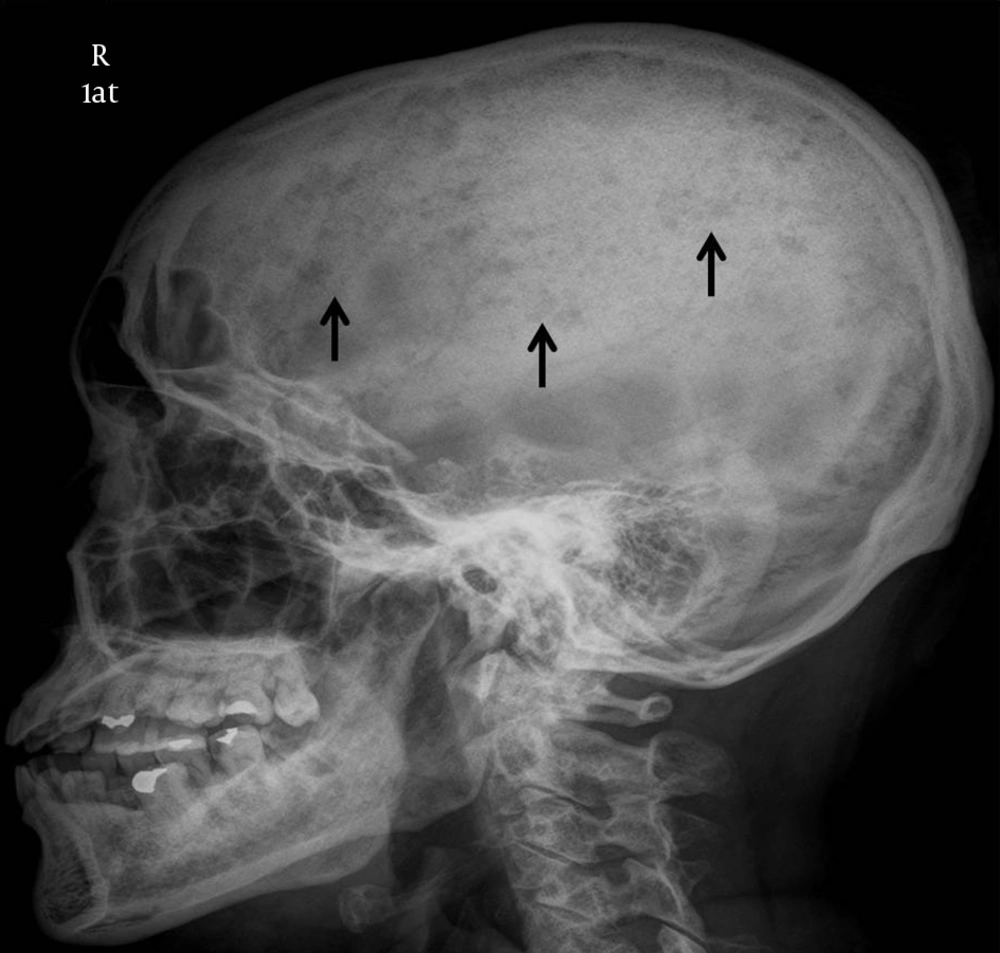
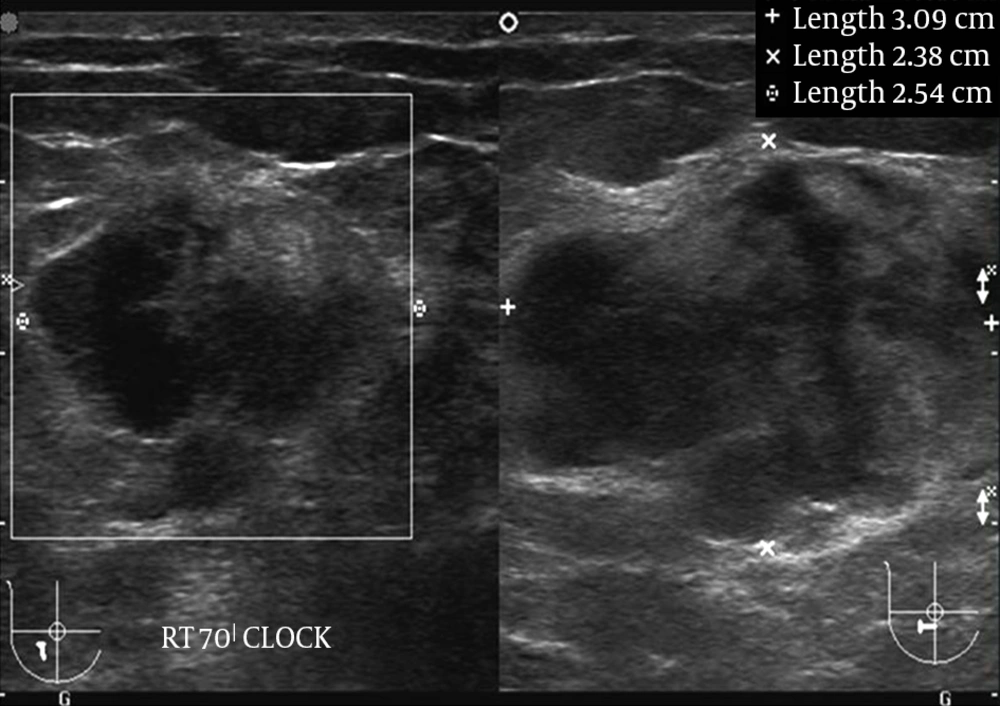
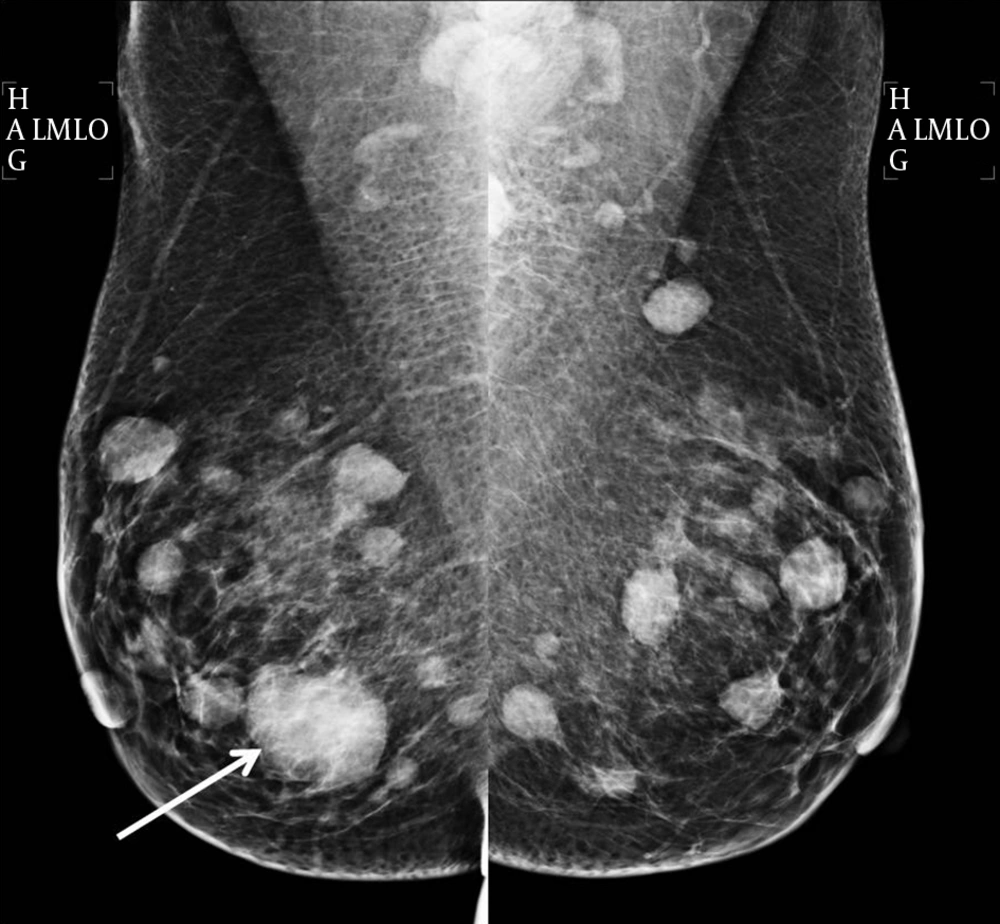
Plain radiography of the right shoulder showed multiple small lytic lesions involving the acromion, clavicle, and humerus (Figure 1). A skeletal survey was performed subsequently and revealed multiple small lytic lesions of the skull (Figure 2). Sonography of bilateral breasts showed multiple lobulated, heterogeneous breast lesions with minimal posterior acoustic enhancement (Figure 3). The sonographic features of the breast lesions were considered to be indeterminate. Mammography (MMG) was performed subsequently; however, only medio-lateral oblique views were obtained in view of the patient’s young age group. There was a dominant mass in the right breast and multiple smaller well-defined high-density nodules within both breasts (Figure 4). As such, an ultrasound-guided core biopsy of the largest lesion was performed the day after mammography. The first diagnosis was primary breast cancer primary with intra-mammary and osseous metastases.
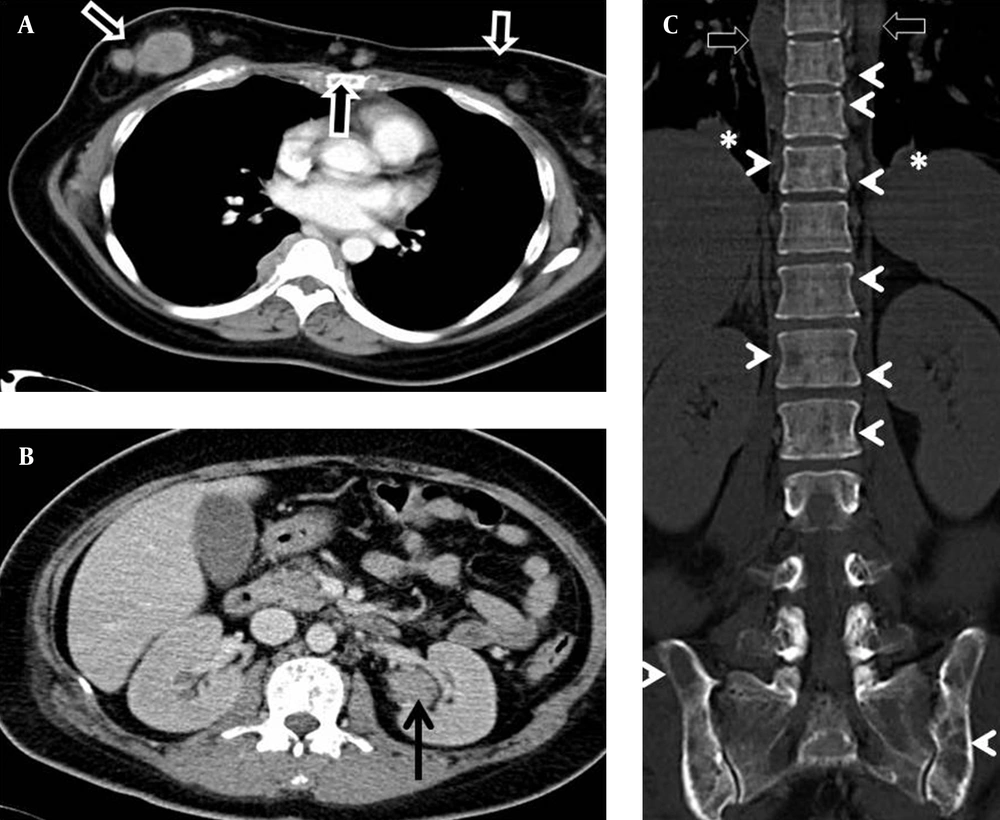
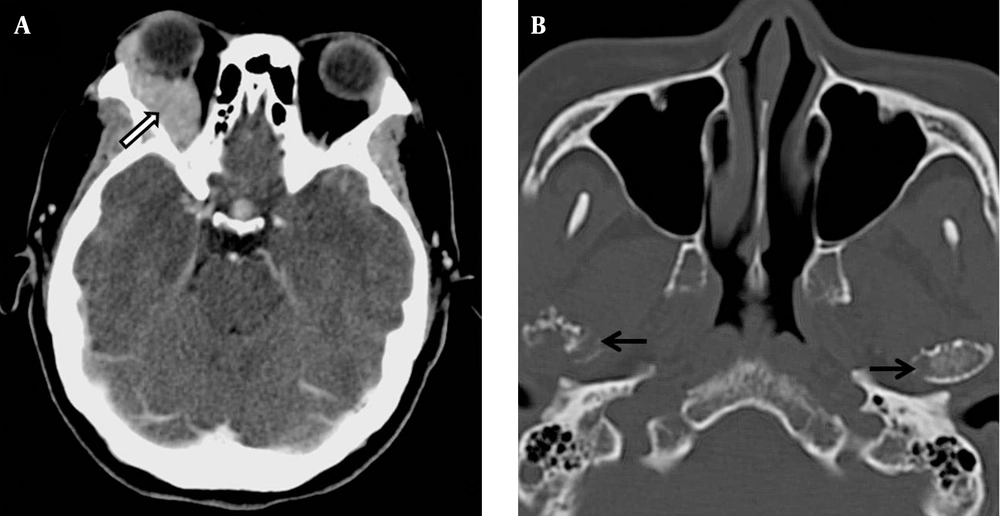
While awaiting the histology report of the breast lump, computed tomography (CT) of the thorax and abdomen was performed to stage the presumed primary breast cancer (Figure 5). This revealed multiple well-defined soft tissue masses involving the subcutaneous tissue, breasts, paraspinal region, left renal pelvis, and lungs. Both kidneys were otherwise normal in size and renal parenchymal thickness. There were extensive lytic bone lesions, some with soft tissue components. Single-photon emission computed tomography (SPECT) bone scan showed no evidence of abnormal radio-pharmaceutical uptake to suggest bone metastases. An orbital CT, performed later due to worsening right eye proptosis, revealed a right extraconal mass. The temporomandibular joints, included in the orbital CT, showed multiple lytic lesions involving both condylar heads (Figure 6). Based on these findings, a rare diagnosis of multiple myeloma with extra-medullary plasmacytoma was considered. She was further investigated with serum and urine electrophoresis, but no monoclonal gammopathy was detected.
Computed tomography (CT) of the thorax and abdomen. A, There are multiple enhancing subcutaneous nodules in the anterior chest wall and breasts (arrows). B, A homogeneous mass in the left renal pelvis not causing hydronephrosis (arrow). C, Multiple lytic bone lesions (arrow heads) with paraspinal masses (open arrow) and lung nodules (asterisk).
Subsequently, the histopathological analysis of the breast lump showed diffuse malignant infiltration by plasma cells, indicating a plasmacytoma of the breast. Bone marrow biopsy was performed and showed more than 80% abnormal plasma cells. Trephine biopsy showed total infiltration by plasma cells suggestive of plasma cell myeloma. A final diagnosis of non-secretory multiple myeloma (Durie-Salmon stage III) with extramedullary disease was made. There was a one-month delay in diagnosis due to the lack of clinical suspicion for this unusual phenomenon and the rarity of non-secretory multiple myeloma.
During the course of the disease, she developed a pathological fracture of the right humerus. Her condition was complicated by hypercalcemia (3.78 mg/dL) and renal failure (serum creatinine of 239 micromol/L) but normalized within 10-day duration after adequate hydration and correction of hypercalcemia. She was started on intravenous bortezomib, cyclophosphamide, dexamethasone, and triple intrathecal chemotherapy. All the EM plasmacytomas completely resolved after the third cycle of chemotheraphy and the Trephine biopsy revealed no excess of plasma cells indicating good chemo-response. She continued another five cycles of chemotherapy and was planned for autologous stem cell transplant. During the course of treatment, she developed a few episodes of neutropenic sepsis and chemo-induced hemorrhagic cystitis. Unfortunately, the disease relapsed one month after the 8th cycle of chemotherapy with multiple new medullary and extramedullary lesions. She developed multiple cranial nerve palsies and spinal cord compression. The result of the lumbar puncture revealed scattered plasma cells in the cerebrospinal fluid. She finally succumbed, 11-month following initial presentation due to aggressive cranial nervous system involvement with leptomeningeal myelomatosis.
3. Discussion
Multiple myeloma is uncommon in young patients. Patients under 40 years of age constitute 2.2% of all multiple myeloma cases, while those younger than 30 years constitute only 0.3% of all cases (4).
Non-secretory multiple myeloma (NSMM) is a rare variant that accounts for 1 to 5% of all cases of multiple myeloma (5). The disease is characterized by the absence of monoclonal gammopathy in serum and urine by immunofixation electrophoresis. NSMM is associated with longer survival, but these patients usually have more advanced disease than those with classic myeloma during initial presentation (6, 7).
NSMM complicated with renal insufficiency is rare in view of absence of light chains in the urine (6). In the present case, we have considered various possible causes of renal impairment. There was no ongoing renal infection, hypotensive episodes or drug related causes. Though renal biopsy was not performed, imaging studies did not show swollen kidneys, which would suggest obstructive nephropathy or plasma cell infiltration. We believe that renal impairment in this patient was caused by dehydration and hypercalcemia, as it improved with hydration and normalization of calcium level.
EM disease of MM is defined as the presence of malignant plasma cells outside the bone marrow, either as soft tissue masses spreading from bone or arising from EM organs. It is not uncommon to see intramedullary myeloma with EM extension as a result of cortical erosion and subsequent spread beyond the periosteum. However, hematogeneous spread of malignant plasma cells to the EM region is less common.This method of spread accounts for 3.4% of cases at diagnosis, 5% at relapse and during the course of disease (2). EM disease can affect any organ and appear as subcutaneous nodules, renal masses, breast lumps, orbital masses, lung, and pleural nodules. The imaging findings are often non-specific and often mimic other disorders.
In the present case, the patient had extensive marrow and EM involvement affecting the left kidney, both breasts, subcutaneous tissue, right orbit, lung, paraspinal region, and lepto-meninges. Owing to the broad differential diagnoses, this patient’s clinical manifestations did cause a diagnostic dilemma. Our first impression was primary breast carcinoma with extensive metastases. However, breast carcinoma with advanced metastases is mostly accompanied by regional or distant lymphadenopathy. Moreover, bone scintigraphy would be expected to show extensive radio-pharmaceutical uptake or “superscan” appearance. In contrast, there was no lymphadenopathy in our patient and her bone scintigraphy was negative for osseous metastases.
Other differential diagnoses that were considered were lymphoma and soft tissue sarcoma with extensive metastases. Again, lymphoma is usually accompanied by lymphadenopathy, which was not seen in this patient. Soft tissue sarcoma should exhibit radio-pharmaceutical uptake.
Bone scintigraphy with Tc-99m is of little value in the evaluation of multiple myeloma due to excessive bone resorption and absence of characteristic osteoblastic activity (8). However, bone scintigraphy played an important role in making the radiological diagnosis in the present case. There are several tumors that are expected to show normal bone scintigraphy. These include multiple myeloma, some anaplastic tumors and pure osteolytic lesions. In the present case, although extensive lytic bone lesions were shown on CT, bone scintigraphy was negative. Mandibular involvement is common in multiple myeloma but rare in bone metastasis. Based on these two reasons, the diagnosis of MM with EM plasmacytoma should be favored. However, the young age of the patient, and the absence of monoclonal gammopathy in the urine and plasma electrophoresis prevented a straightforward initial diagnosis.
Furthermore, EM disease is associated with younger age, male gender, non-secretory subtype, advanced stage of disease (Durie-Salmon stage III), and extensive bone disease (9). In contrast to NSMM, EM disease at the time of diagnosis shows a significantly poorer survival rate and shorter period of progression-free survival (9).
In conclusion, NSMM with extensive EM involvement in young patients is difficult to diagnose owing to its low incidence and scant biochemical markers. The imaging features are non-specific and may mimic many conditions. However, in the case of extensive lytic bone lesions with mandibular involvement and negative bone scintigraphy, one should consider plasma cell related neoplasm as a possible diagnosis.