1. Background
Bone cement has been extensively used since the early 1960’s, when Charnley introduced it into hip joint replacement surgery, for fixing prostheses (1). Hypotension, pulmonary hypertension and oxygen desaturation are complications often reported during cemented hip arthroplasty and can even lead to cardiac arrest in 0.6 - 1% of patients (2). This series of symptoms is known as the bone cement implantation syndrome (BCIS). Morbidity associated with cementation has been one of the important factors that have led to the growth of uncemented prosthesis implantation in the last years. However, despite these advantages of the uncemented technique over the cemented technique, a significant number of surgeons still like to use cemented prosthesis, based on their experience of better long-term outcomes with cemented total hip arthroplasty. Studies evaluating cemented fixation have reported advantages, such as better prosthesis fixation, lower osteolysis incidence, shorter length of stay and lower expenses (3). For this reason, BCIS and its prevention have constantly attracted the attention of scientists and orthopedic surgeons (4-7).
Various theories have been postulated to define the underlying mechanisms that lead to development of BCIS. The most accepted theories involve diffuse pulmonary microembolisation, induced by extrusion of air, fat, bone marrow and thrombokinase into the femoral venous channels by the expanding and pressurizing bone cement, as the cause of development of BCIS (6-8). Inferior vena cava filters (IVCF) are, at present, extensively used to prevent pulmonary embolism (PE) of venous origin, in patients with high risks (9, 10). Previously, clinicians have tried to implant IVCF and found that they could limit the size of emboli extruded to the right heart chambers through the intraosseous veins (11). Evidence from animal studies supporting the role of IVCF in the prevention of BCIS (12).
2. Objectives
The present study was designed to evaluate the prevention of IVCF against BCIS in more detail, and to establish the role of PE in the genesis of BCIS.
3. Materials and Methods
3.1. Animals and randomization
Fifteen male sheep weighing 25 - 30 kg were supplied by the experimental animal center of the Fourth Military Medical University, Xi’an, China. The experimental procedure has been approved by the Committee of Ethics and Experimental Animal Administration of the Fourth Military Medical University, Xi’an, China.
The 15 sheep were divided into three groups: bone cement free (BCF) group, cement implantation (CI) group and IVCF group. In all the three groups, an osteotomy proximal to the greater trochanter of left femur was carried out before the bone cement packing and IVCF implantation. The femoral canal was not reamed out or packed with any bone cement in the BCF group, whereas the left femoral canals of sheep were packed with the cement in CI and IVCF groups to simulate the cementing procedures carried out in hip replacement. In IVCF group, an OptEase® filter (Cordis Corporation, Freemont, CA, USA) was placed and released in inferior vena cava via the internal jugular vein, under X-ray fluoroscopic machine guidance, prior to packing the cement in the femoral canal, while the IVCF was placed in inferior vena cava without releasing in the CI group.
3.2. Anesthesia
The animals had the vaccinations and deparasitation required for their species and had not been used in any other experimental study. Six hours before the operation, they were transferred to the stables of the minimally invasive techniques unit of Xijing hospital (Xi’an, China), where they were put into individual boxes and their general state of health was confirmed to be satisfactory. All the sheep received general anesthesia throughout all the procedures, using the following anesthetic protocol: a) an intramuscular injection of xylazine (0.1 mg/kg), as preanesthetic medication; b) an intravenous injection of ketamine (4 mg/kg) into the cephalic or tarsal vein; c) after intubation, maintenance of anesthesia with 2 - 2.5% isoflurane in oxygen; and d) insertion of a nasogastric tube to avoid aspiration and possible lesions due to material that might be regurgitated. Monitoring of the heart and respiratory rates, oxygen saturation, and electrocardiogram were performed in all animals with the HP3000 ultrasonic cardiogram equipment (Hewlett Packard, Palo Alto, CA, USA).
3.3. Transfusion and monitoring
The right jugular vein was cannulated and connected with a venous transfusion pathway and pressure sensor by a three-way connector. Lactated Ringer's solution was transfused at the rate of 2 - 4 mL/min. The central venous pressure was monitored at a 5 min interval during experiments. The right femoral artery was cannulated for arterial blood pressure (BP) monitoring and heart rate recording.
3.4. Biochemical Measurements
Arterial blood samples were obtained from the aforementioned BP monitoring catheter before and after bone cement manipulation at a 5 min interval in the CI group, or equivalent time points in the BCF group. Oxygen saturation (SaO2), partial pressure of carbon dioxide (PaCO2) and pH were then measured by ABL Flex80 blood gas analyzer (Radiometer, Brønshøj, Denmark) for these samples.
3.5. Implantation of Inferior Vena Cava Filter
The devices were introduced percutaneously into the inferior vena cava via the internal jugular vein under X-ray fluoroscopic machine guidance (Siemens, Munich, Germany). All the operations were performed in the minimally invasive techniques unit. Cavography (C-arm, Philips V-29, Philips, Hamburg, Germany) using 30 mL of iopamidol-370 (Bracco Diagnostics Inc., Milan, Italy) was performed before insertion of the filter. All the filters were inserted using an 8.5 F introducer, carefully following the manufacturer's instructions, which have been described by other researchers.
3.6. Cement Implantation Model
The proximal end of the femur was exposed and an osteotomy was performed, proximal to the greater trochanter, to permit reaming of the medullary cavity. Through a drill hole in the cortex of the distal one-third of the femur, a snugly fitting 15-gauge short beveled needle was introduced into the medullar cavity. The needle was then connected to a Statham pressure transducer by means of rigid tubing filled with saline, in order to record the medullary pressures. Approximately half the length of the medullary cavity was reamed out and 1 - 2 milliliters of type III acrylic resin bone cement for injection (Tianjin Synthetic Material Research Institute, Tianjin, China) was injected into the cavity. The intra-cavity was pressurized by a stick, simulating a clinically used prosthesis, and indices of BCIS were investigated. In BCIS group, the bone marrow cavity was pressurized to 300 mmHg, within 5 minutes, and the pressure was held at 300 mmHg, for 25 min.
3.7. Statistics
All values are expressed as mean ± SD. Data were analyzed by one-way analysis of variance (ANOVA) followed by the Student–Newman–Keuls test for multiple comparisons (Sigma Stat, Jandel, San Rafael, CA, USA). A P value < 0.05 was considered significant.
4. Results
4.1. Establishment of Bone Cement Implantation Syndrome Model
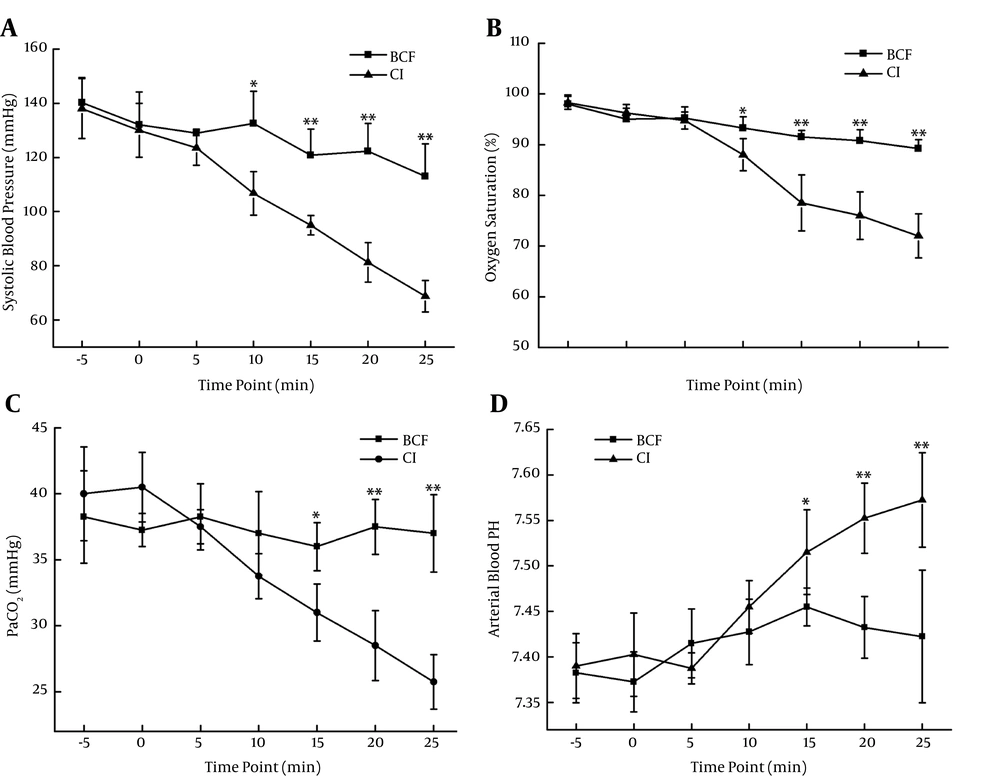
Systolic blood pressure (SBP), SaO2, PaCO2 and arterial blood pH were determined to evaluate the establishment of BCIS (Figure 1). In the CI group, SBP began to decline significantly 10 min after the bone cement was stuffed into the medullary cavity. The SBP changed from 138.0 ± 11.1 mmHg to 81.3 ± 7.3 mmHg, at 20 min, and 7.57±0.05 mmHg, at 25 min, which were significantly lower than the parallel time point in the BCF group. The SaO2 dropped from 98.3 ± 1.3% to 76.0 ± 4.7%, at 20 min, and 72 ± 4.3 mmHg, at 25 min, and PaCO2 declined from 40.0 ± 3.6 mmHg to 28.5 ± 2.6 mmHg, at 20 min, and 25.8 ± 2.1 mmHg, at 25 min, suggesting that type I respiratory failure occurred. The arterial blood pH climbed from 7.39±0.04 to 7.55±0.04, at 20 min, and 7.57±0.05, at 25 min, suggesting that respiratory alkalosis occurred.
Establishment of bone cement implantation syndrome. In bone cement free group, femur osteotomy was performed and the medullary cavity was reamed without bone cement stuffing in experimental sheep. In bone cement implantation group, 2 mL of type III acrylic resin bone cement was injected into the medullary cavity, after the cavity reaming. The intra-cavity pressure was raised to 300 mmHg and maintained for at least 25 min. Systolic blood pressure, arterial oxygen saturation, partial pressure of carbon dioxide and arterial blood pH was measured at the indicated time point. * P < 0.05, ** P < 0.01.
4.2. The Preventive Effects of Inferior Vena Cava Filters on Bone Cement Implantation Syndrome
To determine the effects of IVCF on BCIS, we compared SBP, SaO2, PaCO2 and arterial blood pH between BCF, CI and IVCF groups, 20 min after the bone marrow cavity was pressurized to 300 mmHg, rapidly. The SBP in CI group was 81.3 ± 7.3 mmHg, significantly lower than the control (BCF, 122.3 ± 10.3 mmHg), and IVCF implantation effectively reversed the decline of SBP after bone cement stuffing. The SaO2 were 90.8 ± 2.2%, 76.0 ± 4.7% and 88.3 ± 1.7% in BCF, BCIS and IVCF groups, respectively, which suggested the hypoxia induced by bone cement application was inhibited by IVCF. Similar effect also occurred in relation to PaCO2, with values of 37.5 ± 2.1 mmHg, 28.5 ± 2.6 mmHg and 34.5 ± 1.5 mmHg in BCF, BCIS and IVCF groups, respectively. For arterial blood pH, IVCF prevented the occurrence of respiratory alkalosis, with a value of 7.47 ± 0.02, which was much lower than that in the BCIS group (7.55 ± 0.04).
Bone cement free, cement implantation and inferior vena cava filter models were prepared as mentioned in Figure 2.
4.3. Preventive Effects of Inferior Vena Cava Filters Against Pulmonary Embolism
On echocardiographic evaluation, no emboli-like echoes were in BCF group throughout the experiment. Uneven dotted echoes in the right atrium of the CI group were observed 5 min after bone cement stuffing. The echoes were exaggerated to snow-flask-like ones 10 min after bone cement stuffing (Figure 3). On contrast, such echoes were invisible, even 25 min after bone cement stuffing, in IVCF group. Autopsy oil red staining demonstrated that multiple fat emboli were present in the lungs of CI group subjects, which were not significant in the BCF and IVCF groups (Figure 4).
The effects of inferior vena cava filter on the thrombus occurrence in atrium, induced by bone cement implantation. The bone cement free (BCF) model and bone cement implantation syndrome model were prepared as mentioned in Figure 1. In the inferior vena cava filter (IVCF) group, IVCF were placed into inferior vena cava under the X-ray monitoring before the CI procedure was performed. Ultrasonography was applied to assess thrombus occurrence in atrium. A. BCF group; B. CI group; C. IVCF group, RA: Right Atrium, RV: Right ventricle, LV: Left Ventricle
The effects of inferior vena cava filter on thrombus occurrence in atrium, induced by bone cement implantation. The bone cement free (BCF) model and cement implantation (CI) model were prepared, as mentioned in Figure 1. In the inferior vena cava filter (IVCF) group, the IVCF were placed into inferior vena cava under X-ray monitoring, before the CI procedure was performed. After the surgery, biopsy of the experimental sheep lung was performed and colored with oil red dying, arrow-indicated region was magnified 2 times, as seen in the framed area. Scale bar, 150 μm arrow-indicated region was magnified 2 times, as seen in the framed area. Scale bar, 150 μm. A. BCF group; B. CI group; C. IVCF group
5. Discussion
Our results demonstrated that IVCF could prevent BCIS effectively, suggesting that PE might be the leading cause of the constellation of symptoms of BCIS.
The most constant manifestations of BCIS include hypotension, oxygen desaturation and pulmonary hypertension. These symptoms were all induced in the present CI model, which suggests that a reliable BCIS model was established for the following experiments. Zhou et al. established BCIS model with dogs, in a recent study (13). Blood pressure (BP) and partial pressure of oxygen (PaO2) were both decreased in their models, which were consistent with our findings. However, partial pressure of carbon dioxide went down and arterial pH went up in our models, which were opposite to what was found in Zhou’s models. They used mechanical ventilation during their anesthesia and this might be the reason why their animals did not show respiratory alkalosis (13).
The mechanism suggested to cause these symptoms mainly involves four theories: the toxic effects of methyl methacrylate monomers, allergic responses, humoral factors, like cytokines and complement components, and PE induced by high temperature and pressure in the medullary cavity (14-18). The leading cause of BCIS symptoms was commonly recognized as the PE. The emboli are composed of bone marrow, fat, bone particles and gas, which enter the inferior vena cava via injured veinlets, after pressurizing the medullary cavity (19-24). The emboli then enter the right heart chambers and pulmonary arteries, where pulmonary artery resistance increases and blood flow is reduced. Consequently, oxygen exchange is reduced, blood desaturates in oxygen, and BP decreases, as a result of hypoxia-induced cardiac depression. A previous study has proved that the peak pressure in the medullary cavity was positively relevant to the number of emboli (12, 25). They inserted bone wax instead of bone cement, into the medullary cavity, and the embolism and cardiovascular responses were aggravated after pressurizing. In this study, we gradually pressurized the medullary cavity to model BCIS. With the pressure increasing, the hypotension, oxygen desaturation and PE were aggravated.
The IVCF is applied in patients who are at high risk of developing PE, secondary to venous thromboembolism venous thromboembolism (VTE). We hypothesized that the use of IVCF could decrease the incidence of BCIS, since PE is also considered to be the major factor behind the development of BCIS. In the CI model group, using echocardiography, emboli inside the right heart chambers was observed when cement was inserted to the cavity, in the CI group. The removed embolus was dotted first and became snowflake-like later, which is consistent with another study that demonstrated the development of PE (26). No significant embolus was observed during osteotomy and reaming, in this study, which is in contrast to another clinical investigations that showed obvious embolism during drilling in femur and acetabular bones (27). However, no significant image of embolus was detected throughout the procedure in the IVCF group, even when the pressure in the medullary cavity was raised to 300 mmHg. Fat could be stained by oil red, which was used to detect fat component in sheep lungs. The findings from fat staining assay, performed during the autopsy of sacrificed sheep, were consistent with echocardiography, revealing the inhibitive effects of IVCF on PE.
In respect to the components of the emboli in BCIS, bone marrow, fat, bone debris, methyl methacrylate methyl methacrylate (MMA) particles were detected in lungs of dead patients (28-30). The IVCF was supposed to inhibit PE in BCIS, and the influences of IVCF on manifestations, other than pulmonary embolism, were also investigated, as a part of this study. The pressure in the medullary cavity was raised to 150 mmHg, 5 min after bone cement insertion, and 300 mmHg at 10 min after insertion. Arterial BP decreased continuously after cement insertion, in BCIS group, while there was no statistically significant drop in IVCF group. Pulse pressure was concomitantly investigated. Pulse pressure dropped very fast after cement insertion, showing a tendency of shock, while IVCF impeded such tendency. These results suggest IVCF could inhibit hypotension during BCIS.
Simultaneously, there was a preventive effect of IVCF on oxygen desaturation during the development of BCIS. The PaO2 decreased the moment cement was implanted, and respiratory failure type I occurred 20 min after cement implantation, in BCIS group. However, IVCF implanting partially slowed down the rate of PaO2 decrease. Although PaO2 dropped in the IVCF group, as well, it did not reach respiratory failure and kept stable 10 min after insertion. The results suggest that the PaO2 decrease was partially caused by PE in BCIS. Besides, pH and PaCO2 were investigated in the present study. The IVCF also showed preventive effects against the devastating changes of pH and PaCO2.
In conclusion, this study had successfully recreated a BCIS model, which was then used to study the effect of IVCF in preventing BCIS. The IVCF was found to successfully prevent the development of BCIS. The results from this study suggest that there may be a role of IVCF in high-risk patients, in whom cemented arthroplasties are performed.