1. Background
Radiography along with clinical examination is the most important diagnostic method for dental caries (1). Diagnosis of approximal caries and choosing the best treatment plan for them are among the most common problems encountered in clinical dentistry (2). Even experienced clinicians have moderate accuracy and expertise in diagnosing proximal caries on a dental radiograph (3, 4). Studies have reported a sensitivity of 0.4 - 0.6 for conventional radiographs in diagnosing dental caries (4). Also, some researchers in UCLA showed that dentists misdiagnosed the depth of lesions up to 40% by using conventional radiography and in 20% of cases they misdiagnosed sound teeth as carious (5). Thus, it is not unusual if different dentists have different judgments about the same radiographs (6, 7). This is not because of their different therapeutic viewpoints but due to different diagnoses of presence or extensiveness of the lesions on the radiographs (7); this difficulty in diagnosis is due to the fact that the eyes tend to smooth the gray shadows (8). Therefore, radiography has some limitations and is unable to reveal the first stage of caries. It also underestimates the size of demineralized area (9). NIH (National Institute of Health) has mentioned the necessity of improving the accuracy of radiographic methods for caries diagnosis (10, 11). During the recent years, advances in digital radiography have made it possible to use caries detector softwares for diagnosing dental caries. These softwares can measure the grey shadows more precisely in the scale of 0-250 (12). Logicon caries detector is new FDA-approved software for this purpose that helps dentists detect proximal caries on intra-oral digital radiographs (13). This software can separate dentin caries that need to be restored from enamel lesions. Logicon can analyze the changes in radiographic density, detect the demineralized parts of the tooth and also determine whether it is enamel or dentin caries (8), but reports on its accuracy are controversial (8, 13).
2. Objectives
In the present study, we decided to design a software in our country for the detection of proximal caries in the posterior teeth (molars and premolars) and determination of depth and position of carious lesions by analyzing digital radiographs.
3. Materials and Methods
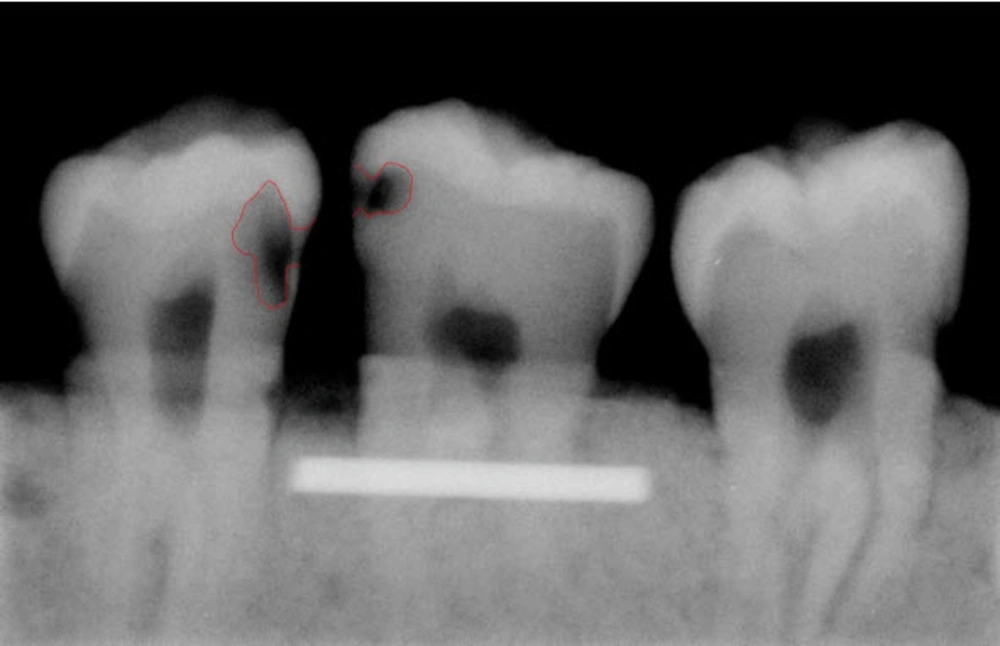
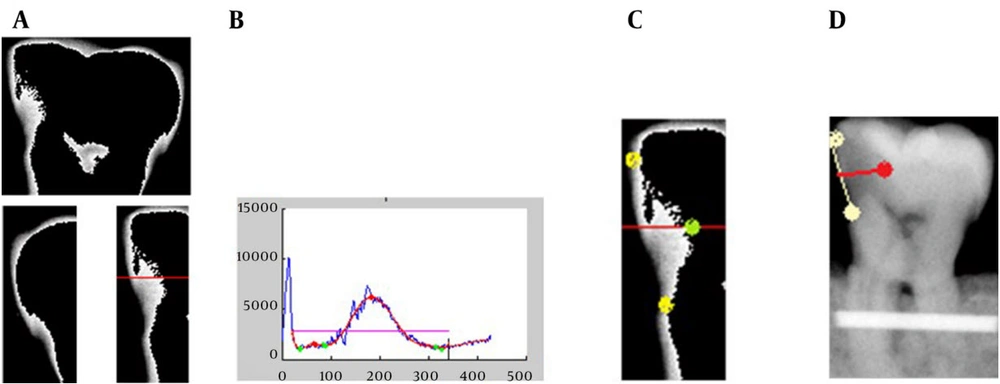
A total of 93 extracted posterior teeth were used in the design phase and 128 in the assessment phase of the software with no restorative materials. None of the teeth had any fracture, or anomalies in shape or size and no gross caries. The teeth had to have intact enamel in the gingival wall of the proximal cavity. The teeth with occlusal, buccal or lingual caries were excluded from the study. Before designing the software, digital radiographs were obtained from proximal surfaces of 183 teeth using Dixi® digital radiographic system (ZV3, Planmeca, Helsinki, Finland). These images were used to train the software designer and to extract basic information. The teeth were stored in 10% formalin solution for more than 24 hours for disinfection and mounted in blocks made of plaster and sawdust in a way that three teeth were placed next to each other with no overlap in their interproximal surfaces. Soft tissue wax up was performed using one layer of 18mm diameter plexiglass sheet and CCD sensor (Dixi, Planmeca, Helsinki, Finland) was placed parallel to the teeth. In order to ensure the reproducibility of the radiographic positioning, the sensor and dental block were placed parallel in a custom-made device for guiding X ray beams, so that the distance of sensor from the source remained constant (25 cm) in all radiographies. Periapical radiographs were taken with Gendex intraoral X ray system (Dentsply, IL, USA, 65kVp, 7 mA), using parallel technique with an exposure time of 0.08. These images were stored in JPEG format. In this phase, the software designer needed some basic information about the shape and form of proximal caries. Routinely, in clinic, when dentists or oral radiologists evaluate dental radiographs in terms of existence and extension of caries, they determine the margin of caries regarding to all radiographic criteria and based on caries extension into the teeth, that appears as a radiolucent area with a density less than tooth structure, and then they decide about the treatment. In this study, for definition and instruction of caries margins to the software designer who was not familiar to definition of dental caries in digitized radiographs, caries margins and the extent of caries were determined manually by drawing a line using Photoshop software by an expert radiologist. We also shared a number of intact caries free teeth with the designer to let him know how the intact surfaces appear in a radiograph, thus reducing the false positive records as much as possible (Figure 1).
3.1. Design Phase
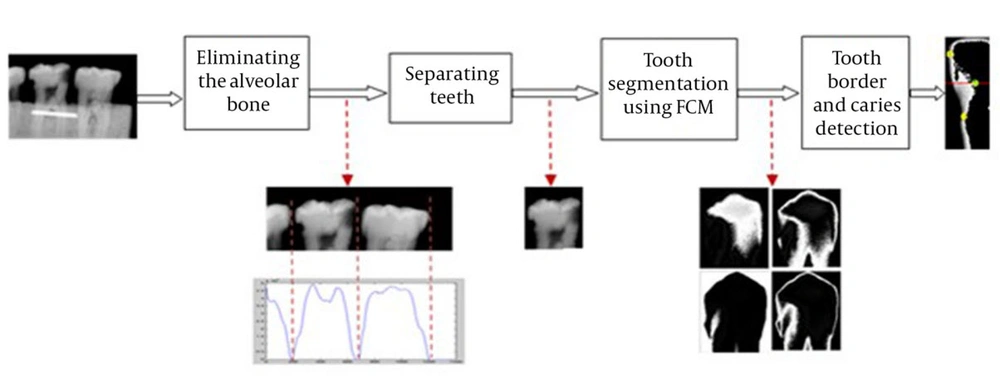
In literature, there are number of researches with focus on automatic caries detection. Several learning algorithms have been used to learn the features and specifications of the caries area. However, as the caries region might have a very small area in various shapes and appearances, no learning algorithm would guarantee a good performance and detection accuracy. Recent researches have examined a new approach called image segmentation to cope with the difficulties of this problem. In computer vision, image segmentation is the process of partitioning a digital image into multiple segments (sets of pixels). The goal of segmentation is to simplify and/or change the representation of an image into something that is more meaningful and easier to analyze. Image segmentation is typically used to locate objects and boundaries (lines, curves, etc.) in images. More precisely, it is the process of assigning a label to every pixel in an image such that pixels with the same label share certain characteristics. As we aim to label the caries area in the image, the designed software basically uses the segmentation technique. The schematic diagram in Figure 2 shows the software’s functionality for automatic caries detection. All the steps demonstrated in the figure are done automatically. In the designed software, we assume that the primary images include one, two or three teeth that are positioned parallel to their longitudinal axis. They should not overlap each other so that the software would be able to distinguish and separate each tooth. By doing so, images entering the caries detection phase would only contain one tooth and therefore, detecting the caries area in them would be more accurate. Separating teeth in the radiography image also makes the caries detection phase independent from the type of the input image, so the image could contain one, two or three teeth. Even if the radiography image has been taken from the upper jaw, a simple pre-processing is required to rotate the image so that teeth in the image are upward. It is worth emphasizing that the designed software can handle cases where teeth in the radiographic image have small overlap in their interproximal surfaces. However, for more accurate results and better software analysis, these cases where excluded from this study. Based on the diagram in Figure 3 the tooth separation phase consists of two steps:
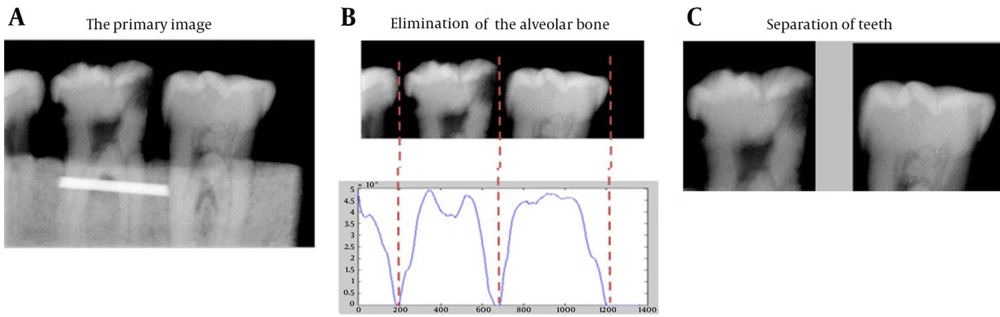
Elimination of the part of tooth located in the alveolar bone (here, the dental block material). The perfect place for cutting the image is chosen based on the summed density of image pixels in each row of the input image (Figure 3 A). As the sum of the density of the pixels (in each row) in the alveolar bone area is much higher than the sum of density of the pixels out of the alveolar bone area, these two sections can be separated automatically with high accuracy using the gradient method. The aim of this part is to remove the alveolar bone section (and the other details that are not helpful for tooth separation) to make the tooth separation phase easier and more accurate.
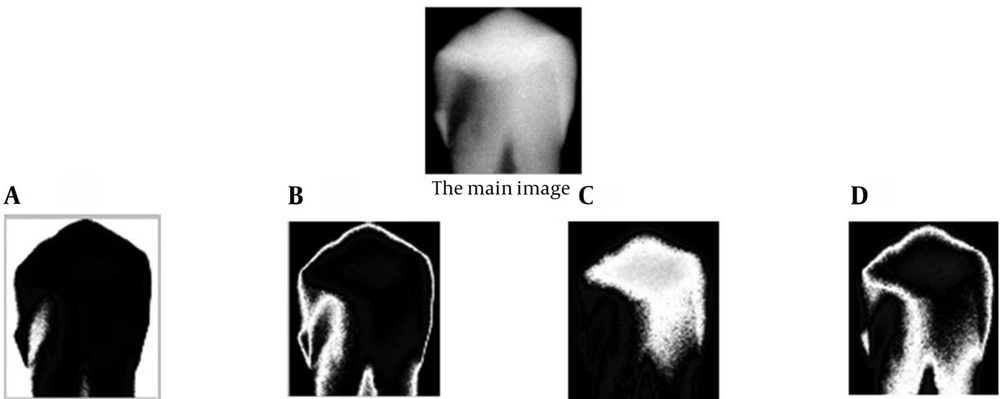
Distinguishing and separating each tooth from the adjacent teeth by using the summed density of image pixels in each column (Figure 3 B). Approximately, a zero-sum density is expected in the column that separates the two teeth, because of the dark pixels in this column of the image. As mentioned before, if the teeth in the image have small overlap with each other, the sum of densities in separating column will not be zero (as it can be seen in Figure 3 B), but it will remain small enough to depict the limit for separation of two adjacent teeth. Therefore, a small and near zero threshold is set for the software to distinguish the separating columns. This threshold can be set automatically using the image size. However, as it is expected, caries detection in images with superimposed teeth would not be that accurate because of the vague border of each tooth. After separation of teeth and elimination of the alveolar bone area from the image, the software tries to segment the tooth into four segments (Figure 4). For this purpose, the FCM (Fuzzy C-Means clustering) algorithm is applied to the image, which has only one tooth. FCM algorithm is a very important tool in image processing for segmenting or clustering objects in an image. It is referred to as a soft clustering method where data elements can belong to more than one cluster, and associated with each element is a set of membership levels.
The greater the belonging of a data element to a cluster, the higher the likelihood of that particular data element to be in the respective cluster. In other words, we believe that image pixels in each cluster have more similar gray levels compared to the pixels that are in other clusters. The nearer the gray level of a pixel to a center of a cluster, the more probable the belonging of that pixel to that cluster. It should be noted that FCM has been selected from a wide range of available algorithms for segmentation and clustering, because of its soft clustering nature. As the densities in different segments of the tooth (specially the caries area) might be very similar to each other, soft clustering brings the opportunity for better caries detection. Also, FCM considers the adjacency of the pixels in each image. As tooth segments (such as dentine and enamel) are supposed to be continuous areas, this algorithm works well for tooth segmenting purpose. In fact, the FCM minimizes the following criteria (Equation 1):
where C and N are the number of clusters (or segments) and pixels respectively, Cj is the center for the j-th cluster, xi is the gray level of the i-th pixel and uijm shows the degree of membership of xi in the cluster j. For each pixel in the image, the cluster with the highest membership degree is considered as the cluster that the pixel belongs to (Figure 4).
In this software, parameter j in FCM algorithm has been set to four experimentally; therefore, each tooth image is segmented to four clusters. After applying FCM, each tooth is divided into four segments. The first segment (Figure 4 A) generally represents the background area of the image and the following segments generally demonstrate the tooth enamel (Figure 4 B), dentin (Figure 4 C) and dental pulp (Figure 4 D), respectively. However, as it can be observed in Figure 4, this categorization is approximately clinical and shows the tooth structure only to some extent, but it can be used for detection of carious lesions. After clustering, a combination of clusters is prepared and entered into the caries detector algorithm.
A curve compatible with the external surface of the tooth border is detected by the software using an edge detection algorithm and presence of caries is examined based on the trend of alterations in the outer border of the tooth (Figure 5). In fact, we assumed that the border of a normal tooth has no extreme (distinct minima or maxima) and the deformation in the border of a healthy tooth is smooth (at least locally). Since cavities in the tooth border curve violate this assumption and can produce big variations in the border curve, this assumption has been used as a natural and logical measure to detect cavity (Figure 5 B). The software detects the local maxima or minima points in the border curve using a gradient method and introduces them as candidates for deepest points of the caries lesion. In order to determine the depth of carious lesions, a line is drawn by the software connecting the two apical and coronal points of a carious lesion (extracted from the curve in Figure 5 B) in the external border of a tooth (Figure 5 D). This line shows the approximate border of the intact tooth in the carious region that is based on our assumption of smooth border for the tooth. By marking the midpoint of this line and connecting it to the deepest point of the carious lesion, a line is obtained whose length is indicative of the depth of the carious lesion (Figure 5 D). In order to convert its unit to mm, a one cm metal index was placed in dental blocks before taking the images, to identify the magnification rate of radiographs (Figure 5 D).
One important point that has been seen in the designed software is that there are various indications for teeth extraction. Even intact teeth may be extracted because of orthodontic requirements to provide enough space for misaligned adjacent teeth. Therefore, the designed software makes no pre-assumption on the teeth health or shape in the input image and every tooth in that image may or may not contain caries. Actually, the software separates teeth in the image and does all the processing phases to investigate each tooth for existing caries. So, this study also contained intact teeth and did not involve carious teeth necessarily.
Since the borders in an intact tooth are expected to be smooth curves with no distinct extreme, the chance of labeling an intact tooth as a tooth with cavity is very small in this software. Therefore, it seems that there is no need for negative state investigation in this classification. As automatic caries detection is a challenging problem in its nature, the designed software has made some assumptions (such as non-overlapped teeth) in its first design. However, the software can be improved to detect caries in harder cases. However, no automatic caries detection software can guarantee high performance in such cases. Actually, the software can be improved for these cases to measure the caries probability with an accuracy percent (e.g. to say that the tooth border has caries with a 1mm depth and 80% accuracy). Also, it is rational to change the software from an automatic one to a semi-automatic software for hard cases, when the software asks for manual expert help in some processing phases to improve the accuracy. After the design phase, 128 teeth were used to assess the software’s function.
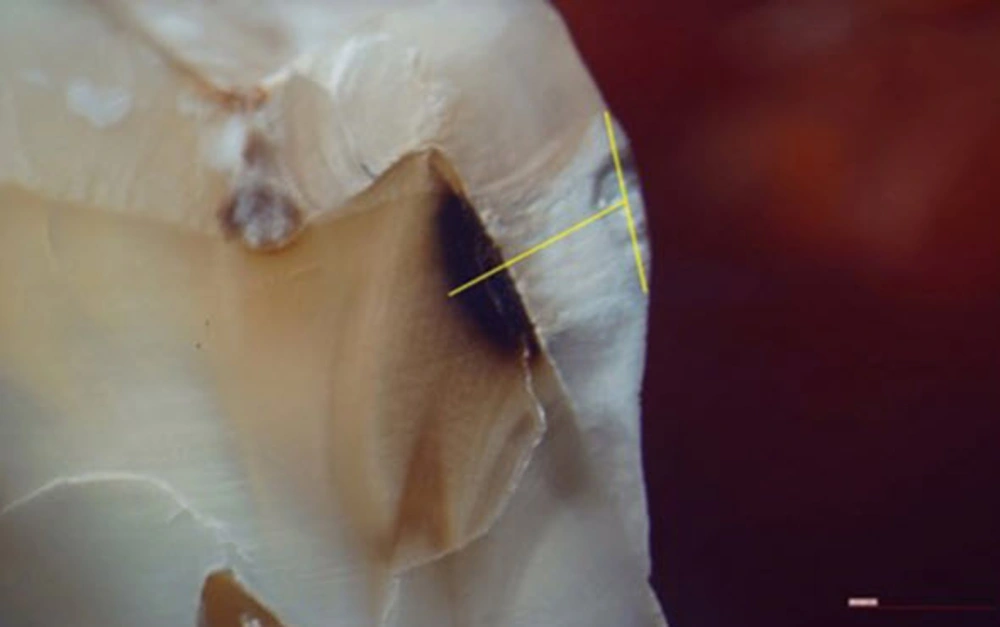
It has to be mentioned that this tooth group was completely different from the first group used for the design phase. The teeth had to be cut in order to determine the exact depth of carious lesions through histological analysis. Thus, the teeth were sectioned mesiodistally along with the central groove of their occlusal surface using a diamond disc (0.15 mm diameter). Intra-rater variability of the above-mentioned data was measured using a single-measure ICC. The results showed an excellent reliability (ICC [95% confidence interval (CI)]: 0.941 [0.913-0.961]). The sections were evaluated by a pathologist using a stereomicroscope (Olympus, SZX9, Japan) with 10× magnification. The criterion for caries diagnosis was the opaque-white to dark-brown discoloration in the caries’ susceptible area. Images were taken in JPEG format from the microscopic views and transferred to the Cygnus media 3-0 software for determining the exact depth of lesions. In this phase, the most coronal point of the external border of the lesion was connected to the most apical point of the external border. Then, the distance of the deepest point of the lesion from the midpoint of the drawn line was measured and defined as the depth of carious lesion in this study (13) (Figure 6). IBM SPSS Statistics 19 for Windows (IBM Corp., Armonk, NY) was used for statistical analysis.
Detection of carious lesions and their depth. A, Upper image is a combination of the segments from the segmenting phase and lower images are mesial and distal borders of the tooth which have been extracted from the upper image by the software. As it can be seen in the image, the distal border has a wide notch, which indicates caries. The increased thickness observed in the mesial border is due to entering the cervical zone, but it does not violate the caries detection aim. B, The curve of tooth border extracted using an edge detection algorithm. The curve has been drawn horizontally for easier interpretation. The deepest point of carious lesion is shown on the curve as the local maximum point which is detected by the software using the gradient method. C, Initial and final points and the deepest point of the carious lesion detected from the curve in part B and shown on the real border of the tooth. D, Measuring the depth of carious lesion assuming a smooth border line for the normal tooth.
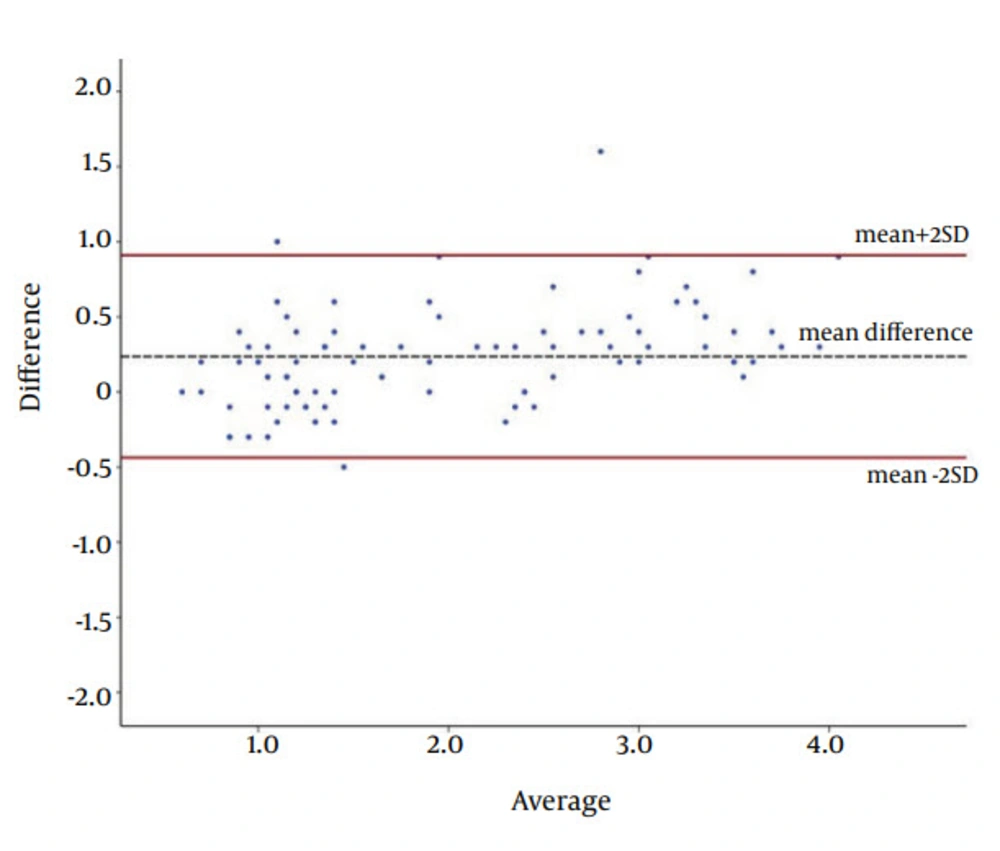
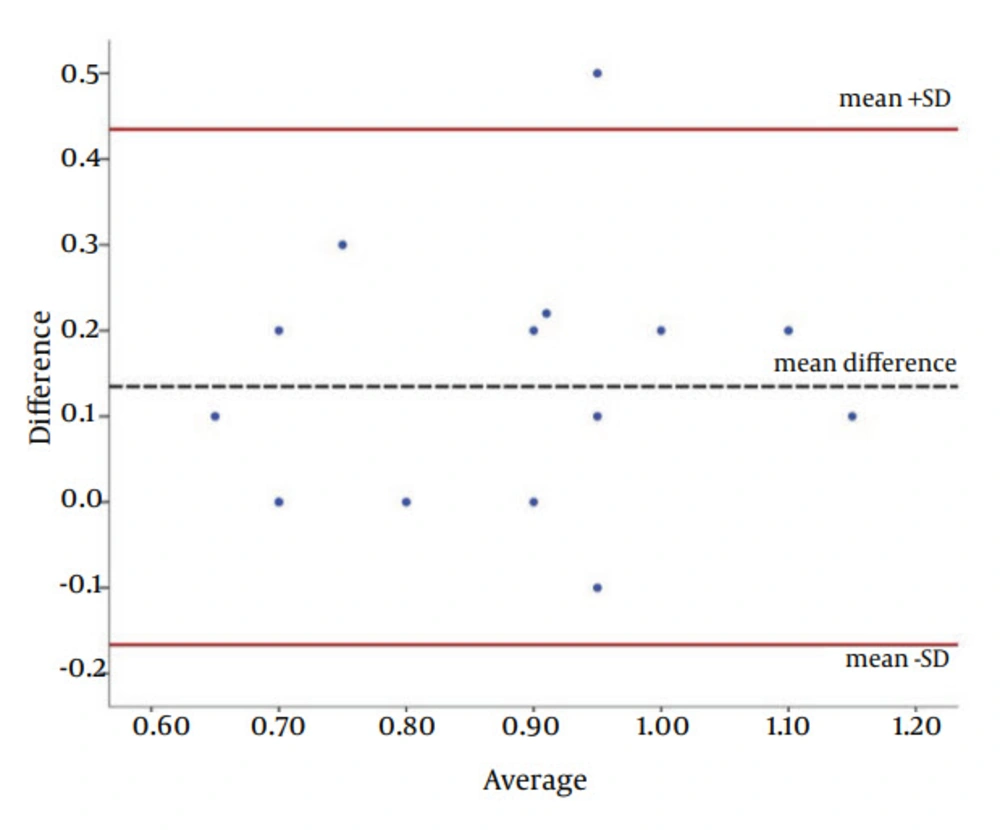
Eventually, intraclass correlation coefficient (ICC) and Bland-Altman plot were used to show the agreement between the software and histopathological analysis (as the gold standard).
4. Results
Of 25 surfaces with enamel caries, the caries detector software was able to diagnose 15 surfaces. In other words, the software diagnosed 60% of enamel caries. The ICC between the software and histological analysis results for detection of enamel caries was determined as 0.609 (95% CI = 0.159-0.849) which was statistically significant (P = 0.006) (Table 1). Also, of 95 proximal surfaces with dentin caries, the software was able to successfully diagnose 93 of them. In other words, the software diagnosed 97% of dentin caries. The ICC between the software and histological analysis results for dentin caries was determined to be 0.937 (95% CI = 0.906-0.958), which was statistically significant (P < 0.001) (Table 1).
The agreement between the results of designed software and histopathology for measuring the depth of caries was investigated by the Bland-Altman plot (Figures 7 and 8). For enamel, only one measurement (4%) and for dentin, four measurements (4.2%) were beyond the acceptable area of the plot (95% CI for mean difference of measurements). This proposed a good and acceptable agreement.
| Location | Number of Proximal Surfaces With Caries | Number of Surfaces Detected With Software | Percentage of Surfaces Detected With Software | ICC | 95% confidence interval for ICC |
|---|---|---|---|---|---|
| Enamel Lesions | 25 surfaces | 15 surfaces | 60% | 0.609 | 0.159-0.849 |
| Dentin Lesions | 95 surfaces | 93 surfaces | 97% | 0.937 | 0.906-0.958 |
5. Discussion
Advances in digital radiography have made it possible to use automated caries detection tools for diagnosing dental caries (8). The previous studies only concentrated on the radiographic diagnostic accuracy of these softwares. However, the International Consensus Workshop in 2002 recommended the application of tools for quantitative assessment of the progression of carious lesions (14-16). Thus, in the present study, we tried to design a software with higher diagnostic accuracy to determine the depth of carious lesions. According to previous studies, the images obtained by Dixi digital radiographic system (Planmeca) are more accurate than those taken by other systems for determining the depth of caries (14). Therefore, Dixi system was used in this study. However, controversy still exists when comparing histological and radiographic depth of carious lesions (14). Since mechanically made carious lesions have greater chance to be diagnosed (14), we decided to establish our study on natural caries; so, histologic measurement of natural caries depth was used as the gold standard.
In the present study, natural caries and histologic measurement of depth was used as the gold standard because the lesions that were caused mechanically had a greater chance to be diagnosed compared to natural ones. In this study, we used teeth with natural caries and because of random sampling, distribution of samples in the two groups of enamel and dentin caries was not similar (95 samples with dentin caries and 25 samples with enamel caries). In the current study, we tried to design the software with the help of MATLAB software. After designing, its function was evaluated and it was revealed that the designed software was able to diagnose 60% of enamel and 97% of dentin caries.
The ICC between the software and histological analysis results was 0.609 for enamel and 0.937 for dentin caries. Thus, we may conclude that the software is more successful in detecting the dentin caries. Also, it is capable of successfully determining the depth of dentin and even enamel caries. These results were acceptable because the literature has shown that caries are not visible on radiographs until they penetrate more than one half the enamel thickness and enamel lesions are usually not visible until 30 - 40% of the lesion has become demineralized (4). That is why the actual depth of penetration of a carious lesion is actually deeper than it appears on the radiographs. Nevertheless, detecting early enamel caries may not be of clinical interest, since not all early enamel caries should be restored and many of these lesions are capable of being remineralized by proper preventive procedures(4). Sometimes, the overlapping in adjacent teeth in the radiographs is a serious problem in caries detection.
In our study, as explained before, the software gets an image with two or three teeth and uses a separation phase to separate the teeth from each other. This is done by summation on the intensities of the image in each column and columns with lower intensity sum are considered as candidates for the place of separation between two teeth. Therefore, in case of small to medium overlap between teeth, the summation remains small enough to indicate the place for separation between the two teeth and the software works properly, although the software accuracy in these cases may decrease. We should note that in usual condition, if a radiograph shows teeth overlapped (that is because of incorrect horizontal angle of X-ray in capturing phase of radiographs from patients), dentist or radiologist diagnosis for caries detection is not reliable and usually the radiographs need to be repeated. So our software is not able to omit the technical errors in taking radiographs.
The results in this study were in accordance with those of Wenzel et al. (13). They compared diagnostic accuracy of Logicon Caries Detector program with that of human observers in approximal caries. They showed that when deeper lesions are the main concern, Logicon can help in diagnosis. In a study by Behere and Lele, the overall accuracy of Logicon Caries Detector program for enamel and dentin caries were 79% and 96%, respectively. However, Logicon appears to be more reliable in ruling out carious lesions than in detecting them whether in the enamel or in dentin (17). In another study, Wenzel et al. (18) found mean kappa value for inter-observer agreement for caries scores to be 0.47 before and 0.48 after the use of LCD, which did not improve using the program. They also found that the program was not consistent and provided diverse opinions on the caries status.
Our findings were also in agreement with those of Gakenheimer et al. (8) They believe that Logicon helps in automatically diagnosis of dentin caries. Dentists’ accuracy improved from 75.6 percent before using the software to 88.3 percent afterward. Tracy et al. (19) showed that nowadays with upgraded CAD software in Logicon caries detectors, it is possible to find twice as much early dentine carious lesions requiring restoration than before, while not needlessly restoring healthy teeth. In their study, the sensitivity was 30% with the initial image and 69% with the density analysis tool. However, the specificity was found to be 97% initially and 94% when the density analysis tool was used (19).
Forner Navarro et al. (20) and Araki et al. (21) reported similar results as well. Forner Navarro et al. found that the Logicon program increased sensitivity, especially in lesions with dentinal caries (20). A similar report by Araki et al. indicates that when cases were restricted to caries in the inner half of the enamel or to dentinal caries, observations with Logicon Caries Detector performed significantly better than without it (21). As mentioned earlier, we used histological analysis results as the gold standard in our study, which is similar to what was done by some researchers in Khon Kean University in Thailand (22). However, they used different statistical indices, which makes it difficult to compare the results. The software designed by researchers in Thailand had a poor function in measuring the depth of lesions, which may be due to the limitations of their Neural Network method for designing.
The diagnostic accuracy of our new designed software is going to be compared with that of human observers and the results will be reported in the near future.The designed software was able to detect a significant number of dentin caries and acceptable measuring the depth of carious lesions in enamel and dentin when compared to the results of histological analysis. However, the software had limited ability in detecting enamel lesions.