1. Background
In patients who have difficulty sitting, thoracentesis is attempted in the supine position (1, 2). A large effusion can be easily approached from the lateral aspect of the hemithorax, such as through the middle axillary line. With a smaller effusion, however, supine thoracentesis can be very difficult (3). The effusion gravitates down to the posterior surface, limiting the space to maneuver the needle-syringe assembly. Recently, Jin et al. proposed a way to circumvent this particular difficulty (4). The authors created gaps at the top of their “Supine thoracentesis bed”, opening a way to approach the hemithorax via the posterior or posterolateral direction in a supine patient.
Although it may be intuitively likely that a posterior or posterolateral approach through a gap in the tabletop would enable tapping of a smaller effusion than a lateral approach, important parameters determining the safety of supine thoracentesis, such as the depth of pleural effusion (DPE) or the distance in a craniocaudal direction that the effusion can be visualized, have not been systematically compared between those three approaches.
In the present study, we compared safety-related parameters between lateral, posterolateral, and posterior approaches in supine thoracentesis, including DPE, the craniocaudal distance (CCD) of effusion with DPE exceeding 1 cm, and the presence of passive atelectasis (which will lessen the chance of pneumothorax following inadvertent lung puncture) in the direction of each approach.
2. Objectives
The purpose of the present study is to compare important safety-related parameters between lateral, posterolateral, and posterior approaches in supine thoracentesis.
3. Materials and Methods
We obtained institutional review board approval for each of the cadaveric experiments and the CT review.
3.1. Cadaver Experiment
Three fresh cadavers (two females and a male; each labeled “Cause of death: old age”), which were donated to our institute for educational and experimental purposes, were available to us. Visual inspection of the cadavers revealed no significant structural abnormalities. One female cadaver was excluded due to the large amount of bilateral pleural fluids detected on ultrasound (US) examination. US of the remaining two cadavers revealed high-echoic, fully expanded right lung and scant amount of pleural fluids.
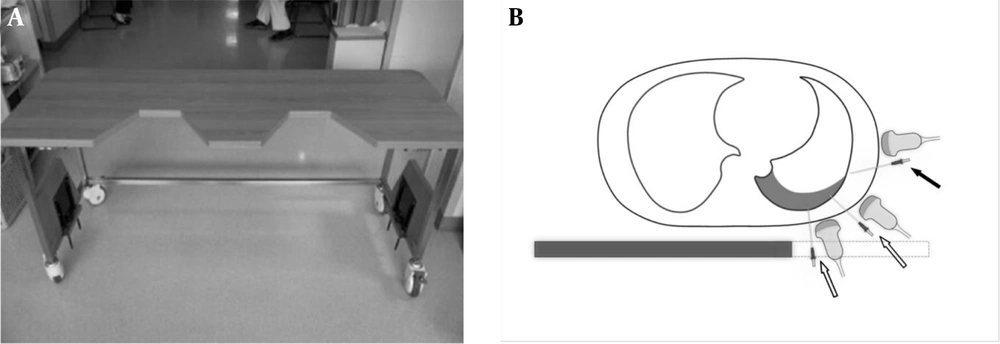
Both cadavers were placed supine onto a table so that the right hemithorax went over a trapezoid gap (20-cm wide, 20-cm long at the medial margin, 30-cm wide at the lateral margin) created at the top (Figure 1). An 18-gauge angiocath needle was inserted to the site of US-detected pleural fluid, until a few milliliters of fluid was aspirated. We gently removed the inner needle and then began administering tap water via the catheter, 100 mL at a time. After each session of water administration, DPE was measured using US at the nipple level at each of the middle axillary lines (with transducer angled horizontally) and the posterior axillary line (with transducer angled 45 degrees to the ground). Water administration was stopped when DPE exceeded 1 cm (the minimum generally considered safe for thoracentesis (5)) at both lines. Statistical analysis was performed using paired Student t-test. P < 0.05 was considered to indicate statistical significance.
A, A table with gaps at one side of the tabletop was constructed for the cadaver experiment. With the lateral margin of the table carved out, the posterior thorax was exposed for the ultrasound probe and tapping needle to approach it from below. B, Drawing of axial cross section in a typical supine patient with a small-to-moderate effusion. Compared to the conventional method of lateral approach (solid arrow), the effusion can be much more easily visualized and tapped from the posterior and posterolateral aspects of the hemithorax (open arrows) via the carved out (dashed line) portion of the novel supine thoracentesis tabulation.
3.2. CT Review
We reviewed all CT scans (n = 4658) obtained between February 2012 and May 2012. In patients with effusion, concurrent lateral-decubitus plain chest radiographs were reviewed to determine whether that effusion was both nonloculated and at least moderate in amount (the width of effusion exceeding 1.5 cm (6)).
CT scans were obtained on one of two CT scanners (LightSpeed VCT, GE Healthcare, Milwaukee, WI, USA; or SOMATOM definition flash, siemens healthcare, Forchheim, Germany), with or without intravenous administration of contrast medium (100 cc at 2 - 2.5 cc/s). All images were reconstructed into axial images with 5-mm slice thickness at 5-mm intervals and coronal images with 3-mm slice thickness.
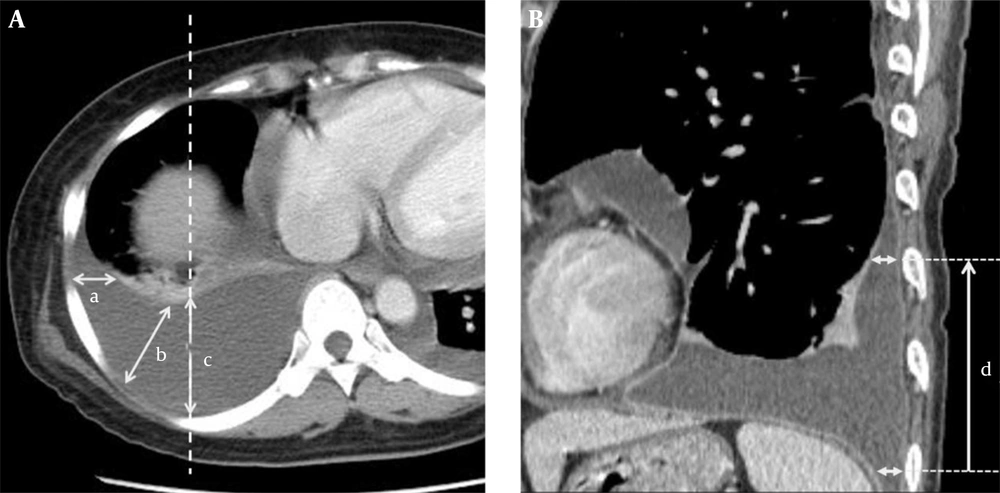
Two radiologists independently and blindly measured DPE and CNR and checked the presence or absence of atelectasis. Interpretation of difference in the presence of atelectasis was determined by consensus. Scrolling up and down axial CT scans, we located the longitudinal position of the greatest DPE in each of the middle axillary lines (lateral approach, horizontal direction), posterior axillary line (posterolateral approach, 45 degrees to the ground), and midclavicular line (posterior approach, posteroanterior direction) (Figure 2A). As the maximal DPE at each approach was measured, the presence or absence of subpleural atelectasis was assessed. At each approach, the CCD was measured by counting the number of axial CT scans showing a DPE greater than 1 cm (Figure 2B).
A, The depths of pleural effusion (DPEs) in the lateral (mid-axillary, a), posterolateral (posterior axillary, b) and posterior (posteroanterior direction to the mid-clavicular line (dashed line, c) tapping routes. The maximal DPEs along the routes a, b, and c may or may not be seen on the same axial plane. B, Nearby the costophrenic sulcus, craniocaudal distance of pleural effusion (d) has been measured (DPE > 1 cm).
Each of the 32 effusions was placed into a small group according to the fluid volume. For each volume group, mean of maximal lateral, posterolateral, and posterior DPEs and CCDs were calculated. In order to assess the effect of effusion volume on the safety of the three approaches, we compared each group’s mean parameter to our safety standards (CCD of 5 cm and DPE of 1 cm). For the estimation of effusion volume, we summed up the slab volumes [5 mm (scan interval of axial CT) × pleural fluid area (manually measured at each axial section scan)] (Figure 3).
For comparisons of DPEs and CCDs, we performed a repeated-measures analysis of variance. When this analysis yielded significant results, it was followed by pairwise comparisons to determine significant differences between the imaginary routes. For comparisons of frequencies of atelectasis, we performed McNemar test. Interobserver variability for measurements of DPEs and CCDs was analyzed by calculating the intraclass correlation coefficient (ICC) with the two-way random effects model. An ICC value lower than 0.4 suggests that the observers are in poor agreement. An ICC value between 0.4 and 0.75 suggests that the level of agreement is fair to good, while an ICC value greater than 0.75 suggests excellent agreement. In all tests, significance was assigned at a P value of less than 0.05.
4. Results
4.1. Cadaver Experiment
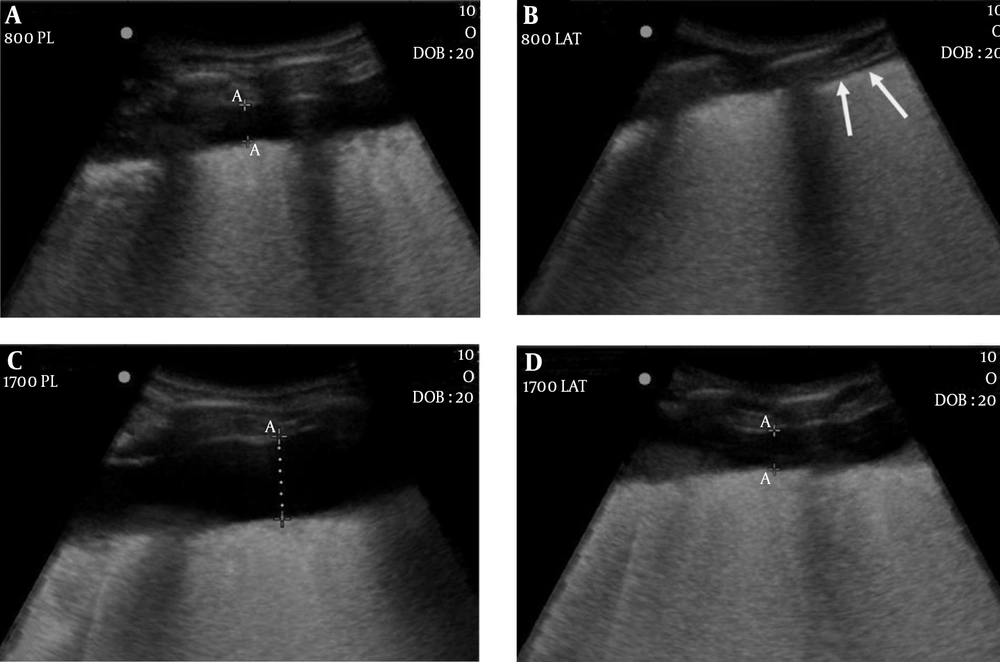
The results of cadaver experiment are shown in Table 1. In each cadaver, DPE in the posterolateral approach was significantly greater than that in the lateral approach regardless of fluid volume (P = 0.002, P < 0.001). In each cadaver, the amount of pleural fluid enough to spread the DPE to higher than 1 cm at the posterolateral approach was less than half that at the lateral approach (500 mL vs. 1,100 mL in Cadaver 1; 800 mL vs. 1700 mL in Cadaver 2) (Figure 4).
| Amount of Administered Water, mL | Cadaver 1, cm | Cadaver 2, cm | ||
|---|---|---|---|---|
| Posterolateral Approach | Lateral Approach | Posterolateral Approach | Lateral Approach | |
| 100 | 0.38 | 0 | 0 | 0 |
| 200 | 0.17 | 0 | 0 | 0 |
| 300 | 0.55 | 0 | 0.54 | 0 |
| 400 | 0.93 | 0 | 0.64 | 0 |
| 500 | 1.14 | 0 | 0.61 | 0 |
| 600 | 1.35 | 0 | 0.71 | 0 |
| 700 | 1.55 | 0 | 0.99 | 0 |
| 800 | 2.87 | 0.2 | 1.12 | 0 |
| 900 | 3.04 | 0.2 | 1.31 | 0 |
| 1000 | 3.44 | 0.22 | 1.34 | 0.1 |
| 1100 | 5.58 | 1.31 | 1.63 | 0.22 |
| 1200 | NA | NA | 1.91 | 0.38 |
| 1300 | NA | NA | 2.29 | 0.57 |
| 1400 | NA | NA | 2.2 | 0.7 |
| 1500 | NA | NA | 2.3 | 0.83 |
| 1600 | NA | NA | 2.3 | 0.96 |
| 1700 | NA | NA | 2.51 | 1.18 |
| P value | 0.002 | 0.002 | < 0.001 | < 0.001 |
Depth of Pleural Effusion in the Cadavers
Cadaver 2. A, Ultrasonography after administration of 800 mL of water shows the depth of pleural effusion (DPE) exceeding 1 cm (1.12 cm) in the posterolateral approach; B, However, there is no fluid gap in the lateral approach (arrows); D, After administration of 1700 mL of water, DPE is slightly greater than 1 cm (1.18 cm); C, While that in the posterolateral approach is much greater than 1 cm (2.51 cm).
4.2. CT Evaluation
Thirty-two hemithoraces (25 patients) meeting the inclusion criteria were identified (Table 2). In the whole subjects, both maximal DPE [1.44 (1.69), 3.69 (1.77), and 4.45 (1.84) cm for lateral, posterolateral, and posterior approaches, respectively] and CCD [4.11 (5.51), 11.50 (5.40), and 18.31 (6.12) cm for lateral, posterolateral, and posterior approaches, respectively] decreased in the order of posterior, posterolateral, and lateral tapping route, with significant difference between them (Table 3). In 13 hemithoraces (40.6%), the maximal DPE was greater than 1 cm in both the posterolateral and posterior approaches but less than 1 cm in the lateral one. At the levels showing maximal DPE, the posterolateral and posterior approaches headed toward an atelectatic lung surface more often than the lateral route (81.3% and 90.6%, versus 28.1%, respectively; P < 0.001 for both comparisons) (Table 3). Interobserver agreement was excellent for all measurements of DPEs and CCDs for lateral, posterolateral and posterior approaches.
| Characteristic | Value |
|---|---|
| No. of patients | 25 |
| Age, y | |
| Mean (SD) | 65.6 (11.8) |
| Range | 18 - 73 |
| Male-to-female ratio | 15:10 |
| Amount of pleural effusion, mL | 667.9 (145.7 - 2019.8) |
| Cause of pleural effusion (no. of patients) | |
| Pulmonary edema | 9 |
| Parapneumonic effusion | 2 |
| Empyema (nontuberculous) | 3 |
| Tuberculous pleurisy | 4 |
| Malignant effusion | 5 |
| Hemothorax | 1 |
| Uremic pleuritis | 1 |
| Distribution of pleural effusion (no. of patients) | |
| Right pleural effusion | 16 |
| Left pleural effusion | 2 |
| Bilateral pleural effusion | 7 |
Characteristics of Study Population
| Lateral Approach, L | Posterolateral Approach, PL | Posterior Approach, P | P Valueb | P Valuec | |||
|---|---|---|---|---|---|---|---|
| L vs. PL | L vs. P | PL vs. P | |||||
| Depth of pleural effusion (DPE)d,e | 1.44 (1.69) (0.974) | 3.69 (1.77) (0.972) | 4.45 (1.84) (0.986) | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| Craniocaudal distance (CCD) of pleural effusiond,e | 4.11 (5.51) (0.990) | 11.50 (5.40) (0.984) | 18.31 (6.12) (0.990) | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| Presence of passive atelectasisf | 28.1 | 81.3 | 90.6 | NA | < 0.001 | < 0.001 | 0.250 |
Results of CT Evaluationa
In the smallest-amount effusion group (estimated fluid volume ≤ 200 mL), the group-average CCD (5.7 (2.8) cm), and the group-average maximal DPE (1.81 (0.16) cm) exceeded our standards only in the posterior route (Table 4). The posterior and posterolateral approaches met our standards in all the other groups. On the other hand, the group-average CCDs of the lateral approach were suboptimal in all but the two largest-effusion groups (estimated fluid volume > 900 mL). The group-average maximal DPE was less than 1 cm in the groups with estimated fluid volumes of 600 mL or smaller. The results for individual effusion have been shown in Table 5.
| Estimated Pleural Fluid, mL | No. of Effusions | Lateral, La | Posterolateral, PLa | Posterior, Pa | |||
|---|---|---|---|---|---|---|---|
| CCD | DPE | CCD | DPE | CCD | DPE | ||
| ≤ 200 | 3 | 0 | 0 | 0 | 0.96 (0.04) | 5.7 (2.8) | 1.81 (0.16) |
| > 200, ≤ 300 | 3 | 0 | 0 | 7.5 (4.6) | 1.82 (0.49) | 11.8 (2.8) | 2.63 (0.58) |
| > 300, ≤ 400 | 4 | 0.8 (1.5) | 0.33 (0.65) | 11.6 (3.2) | 2.38 (0.37) | 17.6 (1.1) | 3.21 (0.51) |
| > 400, ≤ 500 | 4 | 2.3 (3.3) | 0.96 (1.29) | 9.6 (4.3 | 3.18 (0.45) | 16.9 (1.9) | 3.55 (0.65) |
| > 500, ≤ 600 | 4 | 1.3 (2.5) | 0.79 (1.05) | 12.8 (2.5) | 3.28 (0.44) | 20.1 (5.2) | 3.76 (0.35) |
| > 600, ≤ 700 | 3 | 2.7 (3.1) | 1.68 (1.15) | 13.7 (1.0) | 4.88 (0.32) | 18.0 (0.9) | 5.63 (0.46) |
| > 700, ≤ 900 | 3 | 4.5 (3.9) | 1.26 (1.24) | 14.5 (6.1) | 4.73 (0.54) | 22.0 (2.5) | 5.47 (0.33) |
| > 900, ≤ 1100 | 3 | 8.5 (5.3) | 3.04 (2.17) | 16.0 (2.6) | 6.07 (1.94) | 24.2 (4.0) | 6.90 (1.25) |
| > 1100 | 5 | 13.5 (4.4) | 3.96 (0.97) | 15.5 (2.0) | 5.47 (1.12) | 24.5 (2.5) | 6.61 (1.32) |
Craniocaudal Distance and Maximal Depth of Pleural Effusion in Each Effusion Group According to the Estimated Amount of Pleural Fluid
| Estimated Pleural Fluid, mL | No. of Effusions | Lateral | Posterolateral | Posterior | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CCD | DPE | Both | CCD | DPE | Both | CCD | DPE | Both | ||
| ≤ 200 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 3 | 2 |
| > 200, ≤ 300 | 3 | 0 | 0 | 0 | 2 | 2 | 2 | 3 | 3 | 3 |
| > 300, ≤ 400 | 4 | 0 | 1 | 0 | 4 | 4 | 4 | 4 | 4 | 4 |
| > 400, ≤ 500 | 4 | 1 | 2 | 1 | 4 | 4 | 4 | 4 | 4 | 4 |
| > 500, ≤ 600 | 4 | 1 | 1 | 1 | 4 | 4 | 4 | 4 | 4 | 4 |
| > 600, ≤ 700 | 3 | 1 | 2 | 1 | 3 | 3 | 3 | 3 | 3 | 3 |
| > 700, ≤ 900 | 3 | 2 | 2 | 2 | 3 | 3 | 3 | 3 | 3 | 3 |
| > 900, ≤ 1100 | 3 | 2 | 3 | 2 | 3 | 3 | 3 | 3 | 3 | 3 |
| > 1100 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
Number of Effusions That Met Our Standard for Safe Supine Thoracentesis in Each Effusion Group, According to the Estimated Volume of Pleural Effusiona
5. Discussion
The results of this study suggest that in supine thoracentesis, the safety margins of the posterolateral and the posterior approaches are greater than those of the conventional lateral approach. The results of the cadaver experiment suggest that the volume of pleural fluid required for a safe (DPE > 1 cm) posterolateral approach is much smaller than (about half) that required for a safe lateral approach. In the CT review, the maximal DPEs in a given effusion were much greater in the newer approaches (4.45 (1.84) cm for the posterior; 3.69 (1.77) cm for the posterolateral; 1.44 (1.69) cm for the lateral). In 40.6% of effusions reviewed on CT (13 of 32; including one with a volume > 800 mL), DPE exceeded 1 cm in the posterolateral and posterior approaches but less than 1 cm in the lateral approach. In addition, the frequencies of subpleural atelectasis, which would lessen the chance of pneumothorax, were higher at the posterolateral and the posterior approaches (81.3 % and 90.6 % vs. 28.1 %).
The greater the CCD is, the easier it is to set the puncture site away from the abdominal organs. The CT-measured CCDs of the posterolateral and the posterior approaches were far greater than that of the lateral approach. First, the overall-average CCDs were greater (18.31 (6.12) cm for the posterior; 11.50 (5.40) cm for the posterolateral; 4.11 (5.51) cm for the lateral). Second, analysis of the volume groups (Table 4) showed that the group-average CCDs exceeded our standard in the posterior approach regardless of the effusion amount. The group-average CCDs exceeded our standard in all but the smallest-volume group. On the contrary, group-average CCDs were very small in six of the nine effusion groups.
Currently, the sitting position is the standard position for thoracentesis. However, the results of the current study show that the safety parameters of supine thoracentesis can be improved if the posterolateral or posterior approach is used. As shown by Jin et al. (4) these new approaches can be made possible.
There are limitations to this study. First, we performed the cadaver experiment in only two cadavers because of an availability issue. However, the results were supplemented with the CT review enrolling larger number of patients. Second, we failed to include the posterior approach in the cadaver experiment due to restriction in cadaver room time.
In conclusion, safety-related parameters of the posterolateral and posterior approaches in supine thoracentesis are far better than that of the conventional lateral approach. A physician who encountered difficulties during supine thoracentesis using a conventional approach should consider using a suspended supine position and a posterolateral or posterior approach.
![For the estimation of pleural fluid volume, we summed up the slab volumes [pleural fluid area (manually measured at each axial section scan) × 5 mm (scan interval of axial CT)]. For the estimation of pleural fluid volume, we summed up the slab volumes [pleural fluid area (manually measured at each axial section scan) × 5 mm (scan interval of axial CT)].](https://services.brieflands.com/cdn/serve/3170b/541d33e6983091152bb9478e1e15f9abbcc57834/iranjradiol-inpress-20919-g003-preview.webp)