1. Introduction
Perivascular epithelioid cell tumors (PEComas) are rare mesenchymal tumors comprised of immunohistochemically and histologically distinctive perivascular epithelioid cells arranged radially around a vascular lumen and are characterized by abundant cytoplasmic periodic acid-Schiff-positive glycogen (1, 2). These cells express melanocytic (melan-A and microphthalmia transcription factor) and smooth muscle (HMB-45 and actin) markers. PEComas include various histologic subtypes of angiomyolipomas, lymphangioleiomyomatosis, clear cell (sugar) and myomelanocytic tumors in addition to sarcoma of the perivascular cells and pigmented melanotic tumors (1). They have been observed in various organs but are most frequently seen in the kidney, uterus and retroperitoneum and less frequently in the gastrointestinal tract or other organs (3, 4). To the best of our knowledge, ovarian PEComa with pulmonary metastasis has not been described previously in radiologic or pathologic terms. We herein report, for the first time, the imaging and histopathologic features of pulmonary metastatic PEComa arising from the ovaries. This study was approved by our institutional review board. The requirement to obtain written informed consent for study participation was waived.
2. Case Presentation
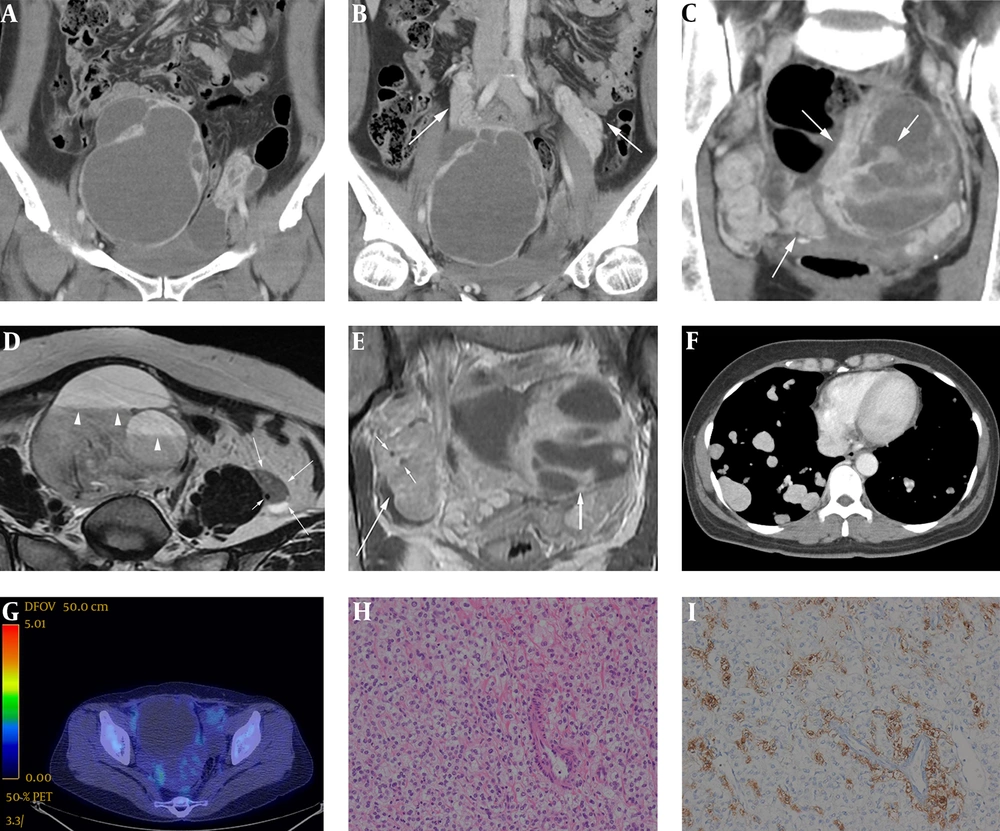
A 48-year-old female presented with mild abdominal discomfort for several months and a history of total hysterectomy for leiomyoma (4 years previously). Physical examination and laboratory findings were unremarkable. Contrast-enhanced abdominal computed tomography (CT) scan was acquired using a 640 slice scanner (Aquilion ONE, Toshiba) by injecting 120 mL of intravenous contrast iohexol (Omnipaque, General Electric Healthcare). CT scans revealed two large, well-defined, multiloculated, solid and cystic masses in the pelvic cavity (Figure 1A). Normal ovaries were not detected, and the small bowel loops and distal colon were displaced by this mass. Therefore, the masses were thought to be originated from both ovaries. Both masses exhibited a thick wall or capsule, multiple internal septations and highly enhancing solid nodules. The highly enhancing solid portions of the pelvic masses encased the bilateral ovarian veins and exhibited a twisted appearance mimicking prominent vascular structures (Figure 1B). The septations and solid portions were highly enhanced (approximately 150 HU on post-contrast images) and exhibited mild washout on the delayed CT scan, on which they were measured approximately 110 HU (Figure 1C). On non-contrast CT scans, the cystic portions of the masses were homogeneously low-density and measured approximately 35-40 HU, suggesting that internal hemorrhage had occurred or that high-density fluid had filled the mass. There was no evidence of internal calcification or a fat component. Pelvic magnetic resonance imaging (MRI) was also performed with a 1.5 Tesla Machine (Signa HDxt, GE Healthcare, Milwaukee, WI, USA), using a body phased-array coil. Bilateral ovarian masses presented as cystic and solid masses. On T1-weighted images, they were heterogeneously hypointense and hyperintense, but the hyperintense portion did not exhibit signal drop on fat-suppressed T1-weighted images, and also showed typical fluid-fluid level with signal decay on T2-weighted axial images, thus indicating internal hemorrhage (Figure 1D). On contrast enhanced T1-weighted images, the solid portions of the masses were highly enhanced. As shown on CT scans, bilateral ovarian vessels were encased by an upwardly extended mass (Figure 1D and 1E) just like twisted bread stick appearance (Figure 2). However, the signal void was well preserved in these involved vessels, so neither vascular flow impairment nor obstruction was evident. Chest contrast-enhanced CT scan revealed multiple, variable-sized, well-defined nodules diffusely distributed throughout both lungs (Figure 1F). These nodules were highly enhanced, measuring approximately 140 HU on post-contrast scans. This prominent contrast enhancement pattern was similar to that of the pelvic cavity masses. No nodules exhibited cavitation or hemorrhage. 18F-fluorodeoxyglucose positron emission tomography/computed tomography (Discovery STE, GE Medical System, Milwaukee, WI, USA) demonstrated mild hypermetabolism within the pelvic and lung masses (maximum standardized uptake value = 2.2 and 2.7, respectively) (Figure 1G). Based on the above imaging findings, rare ovarian neoplasm with perivascular extension along both gonadal vessels was suggested as a differential diagnosis. We included epithelial origin malignancies such as clear cell carcinoma and non-epithelial origin sex cord tumors such as ovarian sclerosing stromal tumor when considering hypervascular solid and cystic adnexal masses. Even though vascular tumors are rare in the female genital tract, particularly in the ovary, vascular tumor such as hemangioma with extravascular growth pattern directly involving the bilateral ovaries could be possible.
A 48-year-old female with malignant perivascular epithelioid cell tumors of the ovaries with pulmonary metastasis. A, Consecutive coronal reformatted contrast-enhanced CT images (A-C) indicated well-defined, solid and cystic masses in the pelvic cavity with highly enhancing internal and peripheral solid portions. B, These solid portions also demonstrated twisted bread stick like appearance due to its wrapping around the bilateral ovarian vessels, mimicking or possibly even representing true vascular structures (arrows). C, These highly enhancing solid portions (arrows) were measured approximately 150 HU on portal phase CT scans, and exhibited mild delayed washout on delayed CT scans (approximately 110 HU). D, On T2-weighted MRI axial scan, as shown on CT, the left ovarian mass with intermediate signal intensity (long arrows) encased left ovarian vein represented as a small signal void dot (short arrow). The ovarian vein can be tracked to the posterolateral aspect of left ovarian mass, where it appears to merge with the mass. Multiple fluid-fluid levels were also observed in the right ovarian mass, suggesting internal hemorrhage and possible hypervascular natured mass (arrowheads). E, On contrast-enhanced T1-weight MR image coronal scan, the right ovarian vessels (short arrows) were also completely encased by highly enhancing, upwardly extending right ovarian mass (long arrow). Neither vascular flow impairment nor obstruction was found as with left side. Large well-defined, solid and cystic mass (thick arrow) with peripheral enhancing solid portion is seen in left pelvic cavity. F, Contrast-enhanced chest CT axial scan demonstrated multiple, variable sized, well-defined nodules diffusely scattered throughout both lungs without regional predominancy. The nodules were highly enhanced, measuring approximately 140 HU on post-contrast scans; such prominent contrast enhancement pattern was similar to that of the pelvic cavity masses. G, Fluorodeoxyglucose positron emission tomography-computed tomography (DG PET-CT) revealed a large pelvic cavity mass with a mildly hypermetabolic solid portion (maximum standardized uptake value = 2.2). This relative photopenia was consistent with the hemorrhagic portion of the mass. H, Photomicrograph (hematoxylin and eosin stain, original magnification, 200 ×) of Perivascular epithelioid cell demonstrated that the ovary masses were composed of predominant epithelioid cells with cleared-out cytoplasm and relatively uniform nuclei. I, Photomicrograph (immunohistochemical stain, original magnification, 200 ×) demonstrated brown particles in the cytoplasm and nucleus of tumor cells, which were HMB - 45 positive.
Bilateral adnexectomy with adhesiolysis and wedge resection of the lung were performed. Intraoperative findings revealed two hemorrhagic cystic masses involving the adnexae. The pelvic cavity masses encased both ovarian vessels. There were severe adhesive changes between the cystic lesions and rectosigmoid colon. Microscopic examination revealed that the tumor was composed of nests of epithelioid cells with cleared-out cytoplasm. Relatively uniform nuclei with mild cytologic atypia were noted (Figure 1H). Immunohistochemical staining revealed that the tumor cells were diffusely positive for smooth muscle actin and strongly multifocally positive for human melanin black-45 (HMB-45) (Figure 1I). Results from histologic and immunohistochemical analyses of tissues obtained from the lung were identical to those of the pelvic masses. The findings were consistent with PEComa in the ovaries and lung.
3. Discussion
PEComa is a rare mesenchymal tumor containing the distinctive perivascular epithelioid cell. The term “perivascular epithelioid cell” (PEC) was first proposed by Bonetti et al. (5) in 1992, and the term PEComa was first introduced by Zamboni et al. (6) 4 years later. This type of tumor consists of three components: thick-walled often hyalinized blood vessels, smooth muscle cells, and adipose tissue. Angiomyolipoma of the kidney and liver, clear cell “sugar” tumor of the lung, and lymphangiomyomatosis are all PEComas (7). There have been approximately 100 cases of PEComas previously reported in the literature, either as case reports or small sample studies. Due to its rarity and the low number of case reports, the most common site for PEComa has not clearly been determined. However, according to a recent study (4), the kidney and uterus are the most prevalent organs. PEComas have a wide spectrum of biological behavior, ranging from benign to malignant. To the best of our knowledge, the present case is the first occurrence of malignant ovarian PEComa with pulmonary metastasis.
Because of the rarity of PEComas, imaging findings of these tumors in various organs are scarce. However, a few recent studies have reported the following common imaging features of PEComas: well-defined border, regular shape, large size without a lobular appearance, complete capsule with a well-defined margin, and frequent mildly necrotic areas (3, 4, 8). The enhancement pattern is typically inhomogeneous, probably due to the heterogeneous composition of the tumor (9). Cui et al. (10) reported a rapid wash-in and slow wash-out pattern of enhancement in renal PEComas. Other reports suggested that arteriovenous hypervascularity in contrast-enhanced CT and MR images is a feature of PEComas (9, 11). Lim et al. (2) recently reported imaging findings for a pulmonary malignant PEComa, which were characterized by well-defined, multiple round nodules exhibiting heterogeneous and intense enhancement (the solid portion was associated with strong enhancement, i.e., 115 HU on post-contrast images). Similar manifestations were also noted in our case. Two large, well-defined, multi-loculated ovarian masses were detected, accompanying multiple septal and peripheral enhancing solid portions that typically showed intense enhancement in the portal phase and slight delayed washout. Although the majority of previously reported PEComas appear to behave in a benign fashion, a malignant course with local recurrence and distant metastasis has also been reported (2, 4, 12). Malignant PEComas are associated with two or more of the following worrisome features: high mitotic rate (> 1/50 high-power fields), atypical mitotic figures, marked pleomorphism and nuclear atypia, hypercellularity, infiltrative growth pattern, large tumor size (> 5 cm), and the presence of necrosis or vascular invasion (8). These features were all observed in our case.
The most notable aspects of our case were that both ovarian vessels were encased or contiguous with the masses. The bilateral pelvic masses contiguously extended along both ovarian vessels and exhibited twisted bread stick like appearance due to their wrapping around these vascular structures. However, vascular obstruction or flow impairment was not evident even though completely surrounded by masses. Despite the large sizes of the bilateral ovarian masses, neither clearly discernible lymphadenopathy nor ascites was observed in the abdominopelvic cavity. Solid metastases to the pulmonary parenchyma alone as the sole form of distant metastatic spread is very unusual without malignant pleural nodules or effusions. The majority of malignant PEComas exhibit no invasion or infiltration of the surrounding structures although contiguous venous invasion has been reported (4).
In conclusion, due to its extreme rarity, an accurate pre-operative diagnosis of ovarian PEComa is thought to be impossible. Little is known about the CT and MRI characteristics of these tumors, which contributes to the difficulty of establishing a diagnosis. However, in cases of ovarian hypervascular masses that encase ovarian vessels, PEComa should be considered as a differential diagnosis.