1. Background
In addition to coronary atherosclerosis, miscellaneous risk factors such as hypercoagulability states, coronary artery spasm, congenital anomalies of coronary arteries, endothelial or microvascular dysfunction, severe ventricular hypertrophy, aortic stenosis, and myocardial bridging (MB) may lead to myocardial ischemia (1). Myocardial bridging (MB) is a congenital coronary artery anomaly. It occurs when a segment of an epicardial coronary artery runs into the myocardial wall, beneath the muscle bridge. This may lead to vessel compression during systole and result in myocardial ischemia.
The most frequent location of MB is in the middle segment of the left anterior descending (LAD) artery (2, 3). The depth and length of MB were reported at a range of 1 to 10 mm and 10 to 30 mm, respectively (4). MB is classified in regard to their depths (superficial: > 1 to 2 mm vs. deep: > 2 mm) (5).
MB was first described by Portman during angiographic imaging in 1960 (6). Since then, coronary arteries have been evaluated by a wide range of newer diagnostic measures such as intracoronary Doppler sonography, magnetic resonance imaging, and coronary computed tomographic angiography (CCTA) (7-11). CCTA has become the primary non-invasive imaging technique for the assessment of MB due to its capabilities such as multi-planar reconstruction, high versatility as well as high accuracy and reliability (12, 13).
As previously mentioned, MB could lead to myocardial ischemia. The occurrence of myocardial ischemia correlates with the length and depth of the bridging vessel, since ischemia is more frequent in long and deep MBs (14). Single photon emission computed tomographic -myocardial perfusion imaging (SPECT-MPI) is a clinical standard imaging technique for myocardial perfusion, but it suffers from poor spatial resolution and soft tissue attenuation artifacts. Also, it has a risk of ionizing radiation inherently (15, 16).
2. Objectives
The aim of this study was to evaluate the potential of CCTA imaging to predict ischemia in patients with MB in comparison with SPECT-MPI imaging.
3. Patients and Methods
3.1. Patients and Image Acquisition
Patients considered for this study were MB-proven by CCTA at Rajaie cardiovascular Medical and research center between March 2015 and March 2016. This study was conducted with an approval from the ethics committee of the Iran University of Medical Sciences. The inclusion criteria of computed tomography angiography (CTA) and SPECT-MPI are described as follows: patients with suspected coronary artery disease who were referred from sub-specialized cardiologists for CCTA and patients diagnosed with MB were selected based on referral to a subspecialized cardiologist to evaluate MB induce ischemia using SPECT-MPI. Patients with active asthma, impaired renal function, drug use which has an effect on the autonomic system, smoking habit, and heart disorders such as coronary artery disease, myocardial hypertrophy, valvular heart disease, and dilated cardiomyopathy, were excluded from this study. Informed consent was obtained from the patients and they underwent stress/rest for SPECT-MPI examination to evaluate ischemic degree.
In order to obtain optimal image quality, the heart rate (HR) of patients was measured before CCTA examination and a heart rate of more than 70 bpm was corrected using 50 to 100 mg metoprolol (Alborz Darou, Iran), which was administered orally. Sublingual nitroglycerin (G.Pohl-Boskamp GmbH and Co, Germany) was also administered for vasodilatation of coronary arteries in order to acquire superior images. All patients were evaluated by Dual-source 384 slice-CT with electrocardiogram (ECG) gated. Contrast administration was executed with a bolus of 30 to 50 cc contrast agent (omnipaque 350 mgI/mL) and a subsequent flow rate of 4.8 to 5.2 mL/s in keeping patient’s weight and glomerular filtration rate (GFR) and using 30 to 40 mL/s of post contrast saline chaser.
Myocardial perfusion study was carried out in a 2-day study protocol. On the first day, a rest study was performed, followed by a stress study (exercise or drug-induced) on the second day. In both rest and stress studies, 740 MBq of Technetium-99m-methoxyisobutylisonitrile (99mTc-MIBI) was injected intravenously and ECG-gated SPECT was started 45 min after injection of 99mTc-MIBI.
CCTA images were acquired from a Dual-source 384 multi-slice CT (Somatom Force, SIEMENS Healthcare, Erlangen, Germany), with prospective ECG-gated. All parameters in CCTA examination are as follows: Collimation width = 384 × 0.6 mm, tube Voltage = 140 kV, tube current = 80 effective mAs tube effective current 330 ms, 500 to 1000 MA, temporal resolution = 66 ms, and slice thickness = 0.6 mm. All reconstructions were done between 30% and 70% of R-R cycle being the best interval for a systole and diastole phenomenon. In one breath-hold during inspiration, scanning was performed from the tracheal carina to the diaphragm. All CCTA images were reconstructed using an iterative technique in short axis, sagittal, coronal and three dimensional (3D) views.
Data acquisition was done using a dual-head SPECT camera system (symbia T2, SIEMENS Healthcare Erlangen, Germany), consisting of a low-energy, high-resolution, parallel-hole collimator; a 20% symmetric window at 140 keV photopeak; a 64 × 64 matrix; an elliptic orbit with step and shoot acquisition at 64 views over 180 degrees rotation. All SPECT-MPIs were reconstructed using filter back projection technique using a Butterworth filter with a cut-off of 0.45 and order of 5.
3.2. Image Interpretation
All CTA images were viewed, characterized, and analyzed on syngo.via software package (Siemens Healthcare, Erlangen, Germany). CTA images were reviewed by a cardiac radiologist with eight years of experience in the field of cardiovascular imaging who was blinded to the SPECT-MPI results. The myocardial features were reported as follows:
1- Muscle bridge location was defined as the “mid part” and “mid to distal part”, according to its relation to arterial branches 1st diagonal artery (D1) and 2nd diagonal artery (D2) respectively.
2- Muscle bridge length was calculated as the distance between the point of entry into the myocardial layer and the point of exit from it.
3- Muscle bridge depth was calculated as the distance between the deepest point of the artery tunnel into the myocardial layer and the surface myocardial layer. MB is classified as regards to their depths: superficial < 2 mm, borderline ~ 2 mm, and deep > 2 mm.
4- LAD was divided into proximal, mid-part, and distal parts according to the diagonal arterial branches. The first part is a segment of LAD before the first diagonal branch, the mid-part is a segment of LAD between the first and second diagonal branches, and the distal part is a segment of LAD after the second diagonal branch. Furthermore, the mid to distal part is defined as a segment of the LAD at the pre to post second diagonal branch.
5- Proximal significant lesion was defined as greater than 50% reduction in diameter of the proximal part of the tunnel artery in the muscle bridge.
6- Diameter of LAD proximal to the muscle bridge (a): was defined as the diameter of the LAD artery before entering the muscle layer (myocardium) at the end of diastole in the short-axis, coronal and sagittal views.
7- Tunnel artery diameter in diastole (b): was defined as the diameter of the tunnel artery measured in the proximal 2 mm from the primary point of entry at the end of the diastole.
8- Tunnel artery diameter in systole (c) was defined as the diameter of the tunnel artery at the narrowest section.
9- Diameter difference was defined as the differences of the LAD diameter proximal to the muscle bridge and tunnel artery diameter in diastole (a - b).
10- Luminal narrowing: (a - c)/a, the luminal diameter of MB was evaluated during the diastolic and systolic phases of the cardiac cycle to determine the effect of systolic compression upon the bridging segments to assume whether ischemia was induced by this luminal narrowing.
11- Systolic compression was defined as (b - c)/b.
12- Muscle index was defined as the multiplication of MB depth by MB length.
The interpretation of SPECT-MPI was done by a nuclear physician with twenty years of experience in this field of cardiac nuclear medicine. The physician was blinded to all CCTA results and a 17-segment model was used. The presence or absence of ischemia, segment involvement and the degree of decreased perfusion for each segment was qualitatively reported as mild, moderate, and severe.
3.3. Statistical Analysis
Numeric data were tested for normality using the Kolmogorov-Smirnov test. The two tailed independent samples t-test or Mann-Whitney U-test were used to compare the numeric data, like age and CCTA based parameters between the two groups. Fisher exact and Chi-square tests were used to evaluate nominal data distribution including sex, MB location, clinical sign, and intensity of ischemia between the two groups. Pearson’s/Spearman’s correlation tests were used to determine correlations between luminal narrowing and MB length (and depth) and between systolic compression index and MB length (and depth). Nominal and numeric data with non-normal distribution have been described as the median and percentage, respectively.
The area under the receiver operating characteristic (ROC) curve (AUC) was calculated for diameter difference, MB depth, and MB length in order to evaluate the overall performance of classification between the ischemia and non-ischemia groups. In this study, a P value of < 0.05 was considered significant and AUC values were estimated beyond the 95% confidence level. Statistical analysis was done using the SPSS software (IBM SPSS Statistics 19 for Windows, IBM Inc., Armonk, NY, USA).
4. Results
4.1. Demographic Data of Patients
This study included 32 patients (21 males and 11 females) with mean age of 48.19 ± 11.70 years. A total of 18 patients suffered from dyspnea while 22 patients had chest pain. Ten patients (31.25%) had both signs of dyspnea and chest pain. MB was more prevalent in the men than in the women and this difference was statistically significant (P = 0.004; Table 1). MB was located in the mid part of LAD in 25 patients (78.1%) and in the mid-to-distal part of LAD in seven patients. Superficial MB was observed in 15 patients, while deep MB and borderline cases occurred in 12 and five patients, respectively (Table 1).
| Variables | Total | Ischemic | Non-Ischemic | P value |
|---|---|---|---|---|
| Gender, No. (%) | 0.004 | |||
| Female | 11 (34.4) | 2 (11.8) | 9 (60) | |
| Male | 21 (65.6) | 15 (88.2) | 6 (40) | |
| MB depth | Superficial, < 2 mm | Borderline, ~ 2mm | Deep, > 2 mm | |
| Frequency, No. | 15 | 5 | 12 | |
| Percent, % | 46.9 | 15.6 | 37.5 |
Abbreviation: MB, Myocardial Bridging.
4.2. CCTA Finding
The distribution of MB anatomic parameters in CCTA images is shown in Table 2. The length and depth of MB, diameter of LAD proximally to MB, tunnel artery diameter in diastole, and systole are 23.25 ± 6.6, 2.30 ± 1.2, 3.30 ± 0.8, 2.40 ± 0.4, and 1.70 ± 0.5, respectively. Also, evaluations of hemodynamic parameters of MB in patients are shown in Table 2. The mean of luminal narrowing, systolic compression, and systolic compression index are 40.3 ± 23.4, 24 ± 18 and 59.3 ± 43, respectively.
| Anatomic measurements | |||||
|---|---|---|---|---|---|
| Length | Depth | Diameter of LAD proximal to MB | Tunnel artery diameter in diastole | Tunnel artery diameter in systole | |
| Mean ± SD | 23.25 ± 6.6 | 2.30 ± 1.2 | 3.30 ± 0.8 | 2.40 ± 0.4 | 1.70 ± 0.5 |
| Minimum | 10 | 1.2 | 2 | 1.5 | 1.1 |
| Maximum | 35 | 5 | 5 | 3.6 | 3.4 |
| Inter-quartile (25 - 75),% | 17 - 30 | 1.5 - 3.5 | 2.5 - 4.0 | 2 - 2.6 | 1.4 - 2.1 |
| Hemodynamic measurements | |||||
| Luminal narrowing, % | Systolic compression, % | Muscle index, mm2 | |||
| Mean ± SD | 40.3 ± 23.4 | 24 ± 18 | 59.3 ± 43 | ||
| Minimum | 0 | 0 | 15 | ||
| Maximum | 75 | 50 | 150 | ||
| Inter-quartile (25 - 75),% | 16.25 - 62.3 | 0.5 - 43 | 30 - 103.7 | ||
Abbreviations: LAD, Left Anterior Descending; MB, Myocardial Bridging; SD, Standard Deviation.
4.3. SPECT-MPI Finding
Of the 32 patients, ischemia was observed in 17 patients (53.1%, 15 males and two females), while 15 patients (46.9%, six males and nine females) did not have signs of ischemia (Table 1). The median age of the ischemic and non-ischemic patients was 45 and 44 years and this difference was not significant (P = 0.583). The locations of ischemia are described in Table 3 In these patients, the most frequent involvements were at the mid septal and anterior left ventricular (LV) walls, as well as the apico-septal and antero-apical walls of the heart. None of these patients presented severe ischemia. While most of the ischemia was mild and moderate, one patient with deep MB had fixed ischemia in the septal region (basal to apical) with the left bundle branch block (LBBB) pattern (Table 3).
| Location | Frequency | Percent in ischemic group | Valid percent | Intensity of ischemia | ||
|---|---|---|---|---|---|---|
| Mild-Moderate | Severe | Fixed | ||||
| Basal | 4 | 12.5 | 12.5 | 3 | 0 | 1 |
| Mid-part | 11 | 64.7 | 34.4 | 10 | 0 | 1 |
| Apical | 10 | 58.8 | 31.3 | 9 | 0 | 1 |
4.4. Compatibility of CCTA and SPECT-MPI Findings
Evaluations were conducted for differences in dyspnea and chest pain in the ischemic and non-ischemic groups and these differences were determined as not statistically significant (P = 0.755 and P = 0.599, respectively) (Table 4). Also, there was a significant difference between the ischemic and non-ischemic groups for MB location and tunnel artery diameter in diastole (P = 0.538 and 0.166, respectively) (Tables 4 and 5). There was a significant difference between the two groups for all other anatomic parameters and all hemodynamic parameters of MB (Table 5). Patients with superficial MB had no ischemia, while ischemia was observed in all patients with MB depth ≥ 2 mm (Figures 1 and 2). In the assessment of CCTA images in patients with MB with LAD arterial proximal segment, significant (> 50% diameter reduction) narrowing is more among patients with positive ischemia as compared to the non-ischemic patients (percentiles 50 positive ischemia vs. non-ischemic patients: 62 vs. 15; P = 0.001) (Table 5). The mean diameter difference after the entry of the artery to the muscle layer was 0.893 ± 0.67 mm. The difference in diameter was found to be significantly different between the two groups (P = 0.001) (Table 5).
| Variables | LAD territory ischemia | Total | P value | |
|---|---|---|---|---|
| No | Yes | |||
| Dyspnea | 0.755 | |||
| No | 7 (46.7) | 7 (41.2) | 14 (43.8) | |
| Yes | 8 (53.3) | 10 (58.8) | 18 (56.3) | |
| CP | 0.599 | |||
| No | 4 (26.7) | 6 (35.3) | 10 (31.3) | |
| Yes | 11 (73.3) | 11 (64.7) | 22 (68.8) | |
| MB location | 0.538 | |||
| Mid Part | 11 (73.3) | 14 (82.4) | 25 (78.1) | |
| Mid-Distal Part | 4 (26.7) | 3 (17.6) | 7 (21.9) | |
Abbreviations: CP, Chest Pain; LAD, Left Anterior Descending; MB, Myocardial Bridging.
aValues are expressed as No. (%).
| MB parameters | Positive ischemia | Negative ischemia | P value | ||
|---|---|---|---|---|---|
| Percentiles, 50 | Percentiles, 25-75 | Percentiles, 50 | Percentiles, 25-75 | ||
| Length, mm | 30 | 22.5 - 30 | 20 | 15 - 25 | 0.002 |
| Depth, mm | 3.5 | 2 - 4 | 1.5 | 1.2 - 1.5 | 0.001 |
| Diameter of LAD proximal to MB, mm | 4 | 3.5 - 4.4 | 2.5 | 2 - 3 | 0.001 |
| Tunnel artery diameter | |||||
| Diastole | 2.5 | 2.2 - 2.8 | 2.2 | 1.8 - 2.5 | 0.166 |
| Systole | 1.5 | 1.3 - 1.8 | 2 | 1.6 - 2.5 | 0.001 |
| Luminal narrowing, % | 62 | 51 - 66.3 | 15 | 10 - 30 | 0.001 |
| Systolic compression, % | 43 | 25.5 - 48 | 6 | 5 - 10 | 0.001 |
| Muscle index | 100 | 55 - 121 | 30 | 18 - 35 | 0.001 |
| Diameter difference | 1 | 10 - 18 | 0.4 | 2 - 5 | 0.001 |
Abbreviations: LAD, left Anterior Descending; MB, Myocardial Bridging.
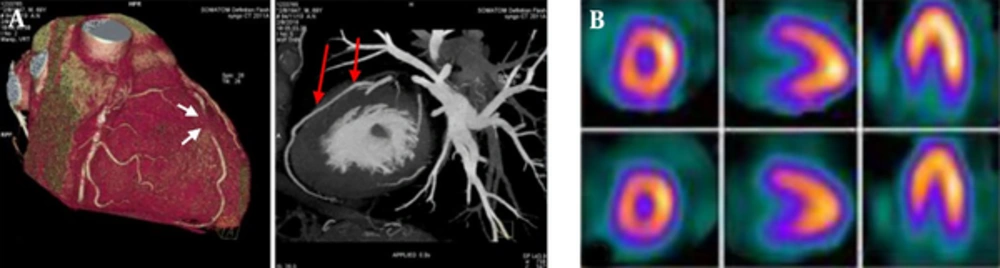
Volume-rendered technique (VRT) and maximum intensity projection (MIP) images show the muscle bridge of the left anterior descending (LAD) by 384 multi slice Siemens somatom force CT angiography (A) in a 70-year-old man with atypical chest pain. The myocardial bridging (MB) is located at the mid segment of LAD with a length of 35 mm and depth of 4 mm (two arrows indicate entrance and exit of MB). The selected slices of the gated SPECT images (B) shows mild to moderate ischemia in the mid anterolateral segment.
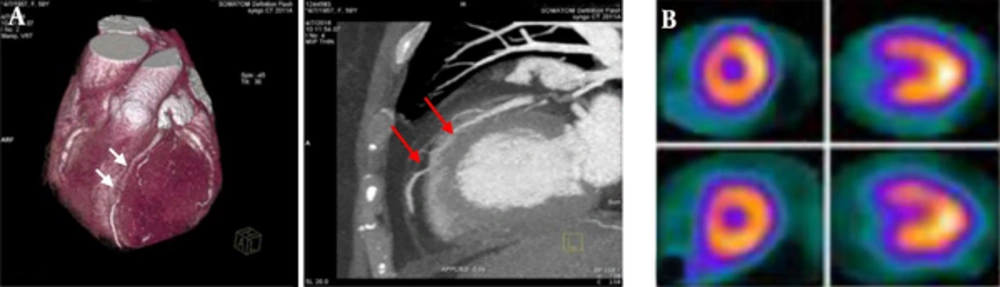
Volume-rendered technique (VRT) and maximum intensity projections (MIP) images show muscle bridge of left anterior descending (LAD) by 384 multi slice Siemens somatom force CT Angiography (A) in 59 years old female with atypical chest pain. The myocardial bridging (MB) is located at mid to distal segment of LAD in length of 20 mm and depth of 2.1 mm (two arrows indicate entrance and exit site of MB). Two selected short and long axis images of the gated SPECT (B) shows reversible perfusion defect in the mid anteroseptal segments.
4.5. Ischemia Prediction Using CCTA Image
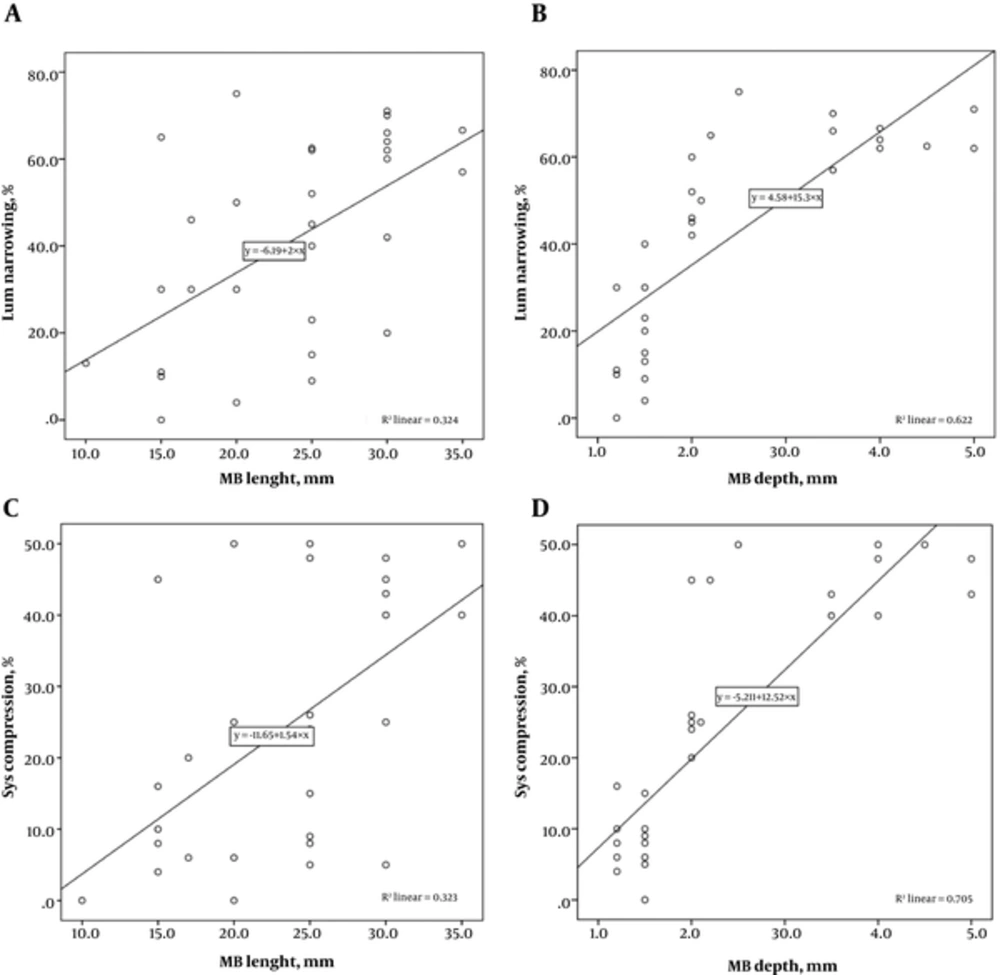
Figures 3A and B show a significant positive correlation between luminal narrowing, MB length and MB depth (r = 0.569, P = 0.001 and r = 0.789, P = 0.001, respectively). In addition, systolic compression showed a significant positive correlation with MB length and MB depth (r = 0.568, P = 0.004 and 0.840, P = 0.001, respectively) (Figure 3C and D).
Scatter plot indicates there are significant positive correlations between: luminal narrowing and myocardial bridging (MB) length with R2 = 0.324 (A); Narrowing and MB depth with R2 = 0.622 (B); Systolic compression and MB length with R2 = 0.323 (C); Systolic compression and MB depth with R2 = 0.705 (D).
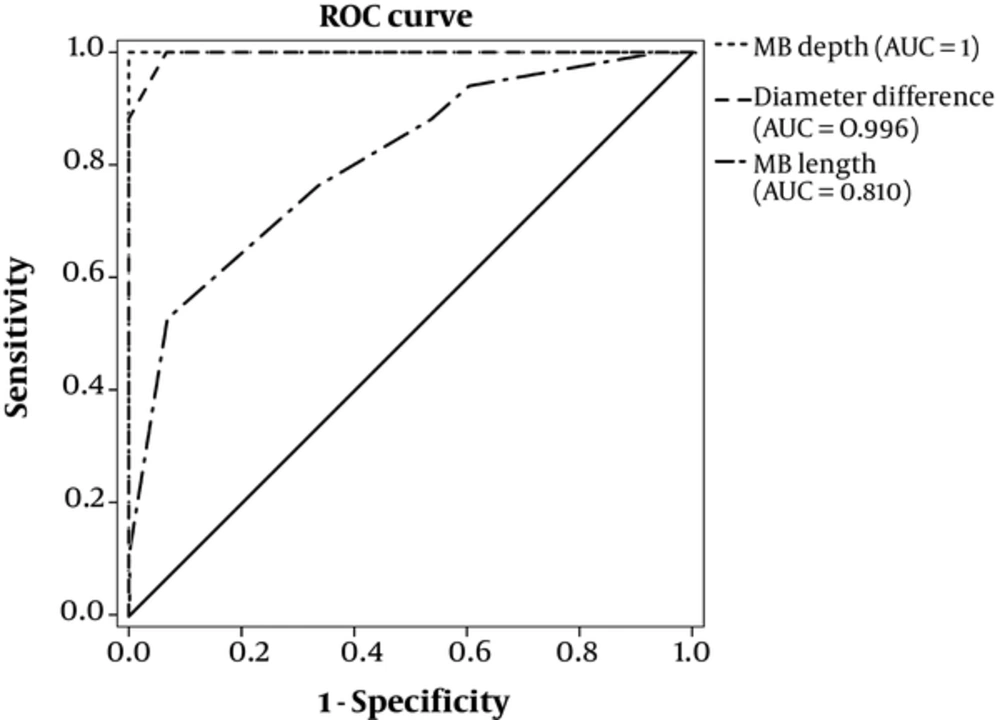
Figure 4 and Table 2 show a ROC analysis of MB parameters in the diagnosis of ischemia. A diameter difference with a cut-off value of 0.65 mm can differentiate ischemic patients from non-ischemic patients with a sensitivity and specificity of 1 (95% CI: 0.99 - 1.00) and 0.93 (95% CI: 0.89 - 0.97), respectively and the area under the ROC curve (AUC) was 0.996 (95% CI: 0.983 - 1.000). Moreover, by considering MB depth cut-off value of 1.75 mm, ischemia can be distinguished with a sensitivity and specificity of 1 (95% CI: 0.99 - 1.00), AUC of 1 (95% CI: 0.99 - 1.00), MB length with 22.5 mm cut-off value had a lower discrimination power with 0.76 (95% CI: 0.60 - 0.92) sensitivity, 67 (95% CI: 0.51 - 0.83) specificity and AUC = 0.810 (95% CI: 0.661, 0.959) in the classification of patients into ischemic and non-ischemic groups (Table 6).
| Variable | Cut-Off, mm | Sensitivity (95% CI) | Specificity (95% CI) | AUC (95% CI) |
|---|---|---|---|---|
| MB depth | 1.75 | 1 (0.99 - 1.00) | 1 (0.99 - 1.00) | 1 (0.991 - 1.000) |
| Diameter difference | 0.65 | 1 (0.99 - 1.00) | 0.93 (0.89 - 0.97) | 0.996 (0.983 - 1.000) |
| MB length | 22.5 | 0.76 (0.60 - 0.92) | 67 (0.51 - 0.83) | 0.810 (0.661 - 0.959) |
Abbreviations: AUC, Area under ROC curve; MB, Myocardial Bridging.
5. Discussion
MB has been considered as a benign condition, but recent studies have shown that it may lead to myocardial ischemia, infarction, coronary spasm, arrhythmias, and finally, death (17-19). Hence, detection of MB could be a critical factor for further reduction of complications and death. In the past decade, studies have shown that CCTA can depict MB with a higher rate than that reported by conventional angiography (19-22). In addition, conventional angiography is an invasive procedure and detecting MBs with significant hemodynamic effect on angiography remains cumbersome (23).
It seems that CCTA can characterize MB location, length, depth, definition of significant LAD arterial proximal segment (< 50% reduction in diameter), narrowing assessment, proximal significant lesion, tunnel artery diameter in diastole and systole, diameter difference, and muscle index. These findings can be used to predict myocardial ischemia. MB depth and length were both shown to be promising predictive parameters for identifying patients with ischemia, as previously discussed. All MB with a depth greater than 1.75 mm had ischemia and MBs with a length greater than 22.5 mm also showed ischemia with an accuracy of 81% (Figure 4). Some efforts have been made to find the correlations between MB length and the presence of ischemia, but the findings remain controversial in the literature. While some studies showed that MB length correlated with ischemia and is in agreement with our results (24-26), other studies reported the opposite (27-29). The reason for the difference obtained in the results of this study compared to others could be related to the newer generation of force CT scan with high spatial and temporal resolution and the iterative reconstruction pattern for better demonstration of superficial from deep MB and its length at CCTA as compared to previous studies by Kim et al. (29) and Niu et al. (27) who used filter back projection reconstruction, as well as Wang et al. (28) who used conventional angiography instead of CCTA for demonstration of MB length as compared to SPECT-MPI. Furthermore, the population differences of patients could be another reason for the differences in MB length induced ischemia at SPECT-MPI in previous studies as compared to the present study.
Furthermore, a diameter difference with a cut-off of 0.65 mm can diagnosis ischemia with 99.6% performance (Figure 4). Accurate and standardized measurement of these MB parameters is of great importance, since it can directly impact the patients’ course of treatment. All patients with deep and long MB and history of ischemia could be considered for medical treatment in conjunction with surgical un-bridging or coronary artery bypass if non-respondent to medical treatment (8). The results of the present study can be of help to physicians in predicting the risk of ischemia and thus, improve the treatment. Moreover, these results can also be of help to prevent unnecessary intervention, noncompulsory treatment, and avoidable complications.
In MB, LAD diameters may reduce after entrance into the myocardia. Although recent studies have mentioned this phenomenon (26, 30), to the best of our knowledge, this is the first study to evaluate the effect of this phenomenon on the prediction of ischemia.
There has been increasing incidence of detection of MB with the advent of the second and recently the third generation of multi-slice CT scanner (31). In this study, Dual-source 384 slice-CT was used to assess MB morphology and the results showed that MB parameters could predict ischemia with high accuracy (Table 6).
Since parameters such as age and clinical signs were not significantly different between the ischemic and non-ischemic patients, these parameters would not act as confounding factors in our study. Also, none of the patients has any underlying disease such as valve disease, atherosclerosis and myocardial hypertrophy that could ultimately affect the results.
There were some limitations in our study. First, the number of patients was small, further investigation using a larger data set would be appropriate. Second, patients were not followed up for a long term and there was no clear picture of how this ischemia-induced test would ultimately result in prognosis.
In conclusion, the results of this study showed that CCTA could help physicians to detect ischemia in MB candidates. Further study may be needed to evaluate the impact of muscle index and luminal narrowing in the prediction of significant hemodynamic MBs.