1. Background
Brown adipose tissue (BAT) as the main organ of regulating energy homeostasis has recently become the focus of interest in the development of new therapeutic strategies targeting BAT proliferation and activation against obesity and related metabolic syndrome such as diabetes mellitus (1, 2). Furthermore, growing evidence advocated a potential role for brown adipose tissue in cancer growth and progression (3-6). Local cross talk between brown adipocyte and tumor microenvironment as well as systemic effect of BAT-derivative cytokines may evolve cell proliferation and angiogenesis as the pivotal step in cancer growth (7-11). Association between brown adipose tissue and mutation in tumor suppressor genes has also been described (7, 12). 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) as the imaging modality of choice to depict in-vivo tissue metabolism has provided major advancement in the field of BAT biology during the past decade. 18F FDG PET/CT studies most of which conducted in cancer patients revealed strong association between BAT recruitment and many intrinsic and environmental stimulators such as cold exposure, lower body mass index (BMI), younger age and female gender. In addition to experimental investigation, positron emission tomography (PET)-based studies have suggested a strong association between breast cancer and BAT activation. There are few reports investigating the potential role of other cancer-related characteristics. One study in pediatric lymphoma concluded that 18F FDG detectable BAT is more prevalent in patients with no evidence of a metabolically active cancer-related lesion (13). Another report indicated that there is no relationship between tumor response and BAT detection rate (14, 15). One investigation has argued that semiquantitive measures of BAT metabolic activity did not correlate with cancer characteristics including cancer type and initial vs. subsequent staging (16). To the best of our knowledge, there is no comprehensive study focused on all aspects of cancer including the potential effect of chemotherapeutic agents in BAT bearing patients.
2. Objectives
The aim of the present study was to investigate the frequency of cancer related characteristics in addition to demographic, anthropometric and seasonal patterns in the population of oncologic patients expressing F-18 FDG detectable BAT as well as to investigate any correlation between the level of BAT metabolic activity, represented by maximum standardized uptake values (SUVmax) as the most validated metabolic parameter in PET/CT study with potential independent determinants.
3. Patients and Methods
3.1. Patients
Of a total 3762 F-18 FDG PET/CT studies performed on oncologic patients in Masih Daneshvari hospital between May 2013 and January 2016, 62 scans were retrospectively identified to have brown fat related 18-F FDG uptake. Fasting blood glucose > 200 mg/dL, insulin administration within 3 hours before F-18 FDG injection, β-blocker and benzodiazepines intake the day before scan and 18-F FDG distribution phase longer than 60 minutes ± 10% were considered as exclusion criteria. Patients’ anthropometric parameters including age, weight and height were recorded. Cancer-related characteristics data including type of cancer and reason for referrals were collected from patients’ medical documentation. BMI, lean body mass and body fat were calculated according to the following formulas and categorized as shown in Table 1: BMI = weight (kg)/ height2 (m), lean body mass for men = (1.1 × weight) -128 (weight/height)2, lean body mass for women = (1.07 × weight) -148 (weight/height)2 and body fat (BF) = weight - lean body mass.
3.2. PET/CT Acquisition Protocol
Whole body F-18 FDG PET/CT was performed using an integrated PET/CT scanner (GE 690 Discovery, 64 Slice, Time of Flight). Fasting period was considered at least 8 and 6 hours before injection for adults and pediatrics, respectively. Blood glucose level was below 150 mg/dL at the time of radiotracer injection. Sixty minutes (± 10%) after intravenous (IV) administration of F-18 FDG (4.6MBq/Kg (0.12 mCi/Kg) for adults and 5.2 MBq/Kg (0.14 mCi/Kg) for pediatrics), CT acquisition commenced craniocaudally from vertex to mid-thigh (or to toe as indicated) with a multidetector CT scanner and the following parameters: auto mAs (adults: 50 - 120, pediatric: 10 - 40), 120 kV, noise factor 19, 2.5 mm thickness. Thirty minutes before imaging acquisition, 40 cc meglumin 76% (containing 370 mg Iodine /cc) in 1500 water was administered as oral contrast in adults. The PET data were then collected in the reverse direction immediately after CT acquisition with a time of 3 minutes per bed position. The PET raw data were corrected for attenuation, dead time, random and scatter coincidence, and subsequently reconstructed by iterative method and high definition (HD) technique. No premedication was administrated before injection for adults and pediatric patients.
| Variables | Value |
|---|---|
| BMI categories | |
| Low | < 19 |
| Normal | 19 - 25 |
| Overweight | 25 - 30 |
| Obese | > 30 |
| Body fat categories, % | |
| Good/acceptable | |
| Male | < 20 |
| Female | < 30 |
| Overweight/obese | |
| Male | > 20 |
| Female | > 30 |
3.3. Image Interpretation and Variables’ Categorization
A team comprised of an experienced radiologist and a nuclear medicine physician reviewed attenuation corrected (AC) and nonattenuation corrected (NAC) PET, CT and fused PET/CT images on advantage window Volume Share 4.5, side-by side and reached consensus for clinical staging, restaging and response to treatment based on image findings and baseline medical records. Disease status was classified as staging (stage 1 - 4 according to the last updated TNM staging system and Ann-Arbor classification, as appropriate), restaging (locoregional recurrence and distant metastasis), and response to treatment (complete metabolic response, partial metabolic response, stable disease, progressive disease, according to positron emission tomography [PET] response criteria in solid tumors [PERCICT] criteria and Deauville score, as appropriate). One to four month interval period after the last course of chemotherapy was considered as status post treatment. Active disease was defined as the presence of hypermetabolic cancer-related lesion located in primary, locoregional or distant sites. Non-active underlying cancer was considered when no locoregional or distant cancer-related lesions were found on PET/CT images either by size criteria or visually-interpreted metabolic activity. Metabolically active malignant lesion was determined based on the size of lesion and FDG avidity of primary tumor by consensus. Hypermetabolic lesions compatible with inflammatory process were not considered as active disease. Active brown adipose tissue was also considered as increased metabolic activity (greater than the level of blood pool activity) on PET images corresponding with fat density on CT images (Hounsfield Unit = -250 - 50) conform the anatomical distribution of brown adipose tissue. SUV max of active brown adipose tissue was measured separately for each anatomical location (i.e. neck, axilla, mediastinum, paravertebrae and abdomen) using volumeshare 4.5 PET VCAR software (GE discovery 690) to semiautomatically delineate the contour of active brown adipose tissue. The level of aortic arch blood pool activity was considered as the cutoff for individual patient.
3.4. Data Analysis
Data analysis was performed using IBM SPSS version 23. Descriptive quantitative variables were expressed as either frequency or mean and range. To test the significant difference between two means, independent T test and Mann-Withney U were used as appropriate. Correlation between average SUVmax and potential influencing factors were analyzed using Pearson correlation coefficients and one-way analysis of variance. Linear regression test was performed for the determinants proved to have significant correlation with average SUVmax based on univariate analysis. P < 0.05 was considered as a statistically significant difference.
4. Results
Of a total 3762 F-18 FDG PET/CT scans conducted for oncologic indications at Masih Daneshvari Hospital between May 2013 and January 2016, 62 (1.6%) scans were retrospectively identified to depict metabolically active brown adipose tissue (1.6%, mean age 29.21 (± 14.18; age range: 6 - 66 years). BAT detection rate was significantly more prevalent in female (42/62, 68% per BAT-bearing patients, 42/1847, 2.27% per total female population) than male (20/62, 32% per BAT-bearing patients, 20/1915, 1.04% per total male population, (P value = 0.007). Table 2 outlines the cohorts’ demographic and anthropometric parameters. Except for pediatrics (< 18 years old), female predominance in BAT visualization was noted in all age groups. There was a trend toward a gender preference in male to depict active BAT in patients < 18 years old; however, it was not statistically meaningful (boys: 7/62 (11.29%), girls: 4/62, (6.45%), P = 0.08).
| Variables | Value | P value |
|---|---|---|
| Gender | ||
| Male | 20 (32.3) | |
| Female | 42 (67.7) | |
| Age, y | 29.21 ± 14.18, 6 - 66 | 0.002 |
| Male | 21.8 ± 7.95, 6 - 65 | |
| Female | 32.82 ± 14.52, 14 - 66 | |
| Weight, kg | 64.17 ± 17.36, 18 - 94 | 0.7 |
| Male | 65 ± 18.8, 18 - 94 | |
| Female | 63.76 ± 14.16, 35 - 94 | |
| Height, cm | 164.6 ± 12.44, 85 - 190 | 0.4 |
| Male | 166.78 ± 17.66, 85 - 190 | |
| Female | 163.53 ± 7.15, 142 - 188 | |
| Body mass index | 23.6 ± 5.27, 15.54 - 33.98 | 0.2 |
| Male | 22.65 ± 5.18, 15.54 - 31.62 | |
| Female | 24.05 ± 5.3, 16.67 - 33.98 | |
| Lean body mass | 46.32 ± 10.81, 4.93 - 68.82 | 0.019 |
| Male | 51.25 ± 11.56, 14.04 - 68.82 | |
| Female | 43.92 ± 6.61, 4.93 - 60.21 | |
| Body fat, % | 17.15 ± 9.48, 2.89 - 39.59 | 0.025 |
| Male | 13.89 ± 7.45, 2.89 - 35.4 | |
| Female | 18.74 ± 7.23, 4.63 - 39.59 |
Abbreviation: y; year
aValues are expressed as No. (%) or mean ± SD, min - max.
Eighty-three point six percent of the patients were < 40 years old. Mean age in patients with activated BAT showed a statistically significant difference between male and female (21.8 (± 7.95) vs. 32.82 (± 14.52), P value = 0.002). None of the BAT-bearing patients >40 years old were male. Ten women > 40 years old (23.8%) expressed active BAT.
Seventy-one point four percent and 88.7% of the patients with active BAT had BMI less than 25 (low BMI: 30.4%, normal BMI: 41.1%) and BF below the obesity cutoff, respectively. The frequency of BAT-bearing patients with normal/low BMI (BMI ≤ 25) (n = 46, 74.19%) was significantly more than those with overweight/obese BMI (BMI > 25) (n = 16, 25.8%) (P value = 0.002). The same results were obtained for BF-based categorical weight (non-overweight patients: n = 59, 95.16%, overweight/obese patients: n = 3, 4.83%, P < 0.001). In addition, there was a significant difference in body fat and lean body mass between male and female groups (P value = 0.027 and P value = 0.002, respectively). However, it was not demonstrated for weight, height and BMI. Notably, gender preference in female was demonstrated in all BMI groups as well as in patients with good/acceptable BF categories. However, in BF-based overweight/obese patients, BAT visualization occurred equally between male and female with the mean age of 25 and 27.75, respectively.
The seasonal and monthly patterns of BAT expression in F-18 FDG PET/CT scan were demonstrated as the following: autumn (47.5%, October: 17.7%, November: 16.1%), winter (21.3%, February: 12.9%, January: 8.1%), summer (17.7%, September: 8.1%, August: 4.8%) and spring (12.9%, April: 8.1%, June: 3.2%) with a statistically significant higher rate of BAT detection in autumn.
In the context of cancer-related characteristics (Table 3), the most frequent brown fat-associated cancers in the current study were lymphoma (Hodgkin’s disease and Non Hodgkin’s lymphoma: 27/62, 43.5%), genitourinary (11/62, 17.7%), and breast cancers (7/62, 11.3%). Most of the patients with active brown adipose tissue were referred for evaluation of response to treatment (30/55; 54.54%), 27 of which demonstrated complete or partial response to treatment (43.54%) followed by metastatic assessment (12/55; 21.81%). In other words, 54.54% of the patients underwent PET/CT investigation in a 1-4-month period after the end of therapy (status post treatment). Thirty five out of 62 patients had at least one metabolically active cancer-related lesion (56.45%).
| Type of cancer | Per BAT patients | Per total patients | Reason for referral | Disease status | Recent history of treatment | Evidence of disease | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lymphoma | 27 (43.6) | 896(3) | Treatment response evaluation | 30 (54.54) | Complete metabolic response | 14 (22.58) | Positive | 27 (43.54) | Positive | 35 (56.45) |
| Genitourinary | 11 (17.7) | 427 (2.5) | Metastatic assessment | 12 (21.81) | Partial metabolic response | 13 (20.9) | Negative | 25 (40.32) | Negative | 27 (43.54) |
| Breast | 7 (11.3) | 468 (1.49) | Restaging | 6 (10.9) | Advanced disease | 4 (6.45) | NOS | 10 (16.12) | ||
| Bone | 3 (4.8) | Staging | 5 (9.09) | Progressive disease | 4 (6.45) | |||||
| Head and neck | 3 (4.8) | Recurrence | 2 (3.63) | Positive for recurrence/ metastasis | 12 (19.35) | |||||
| Adrenal | 2 (3.2) | NOS | 7 | Negative for recurrence metastasis | 7 (11.3) | |||||
| Lung | 2 (3.2) | No active lesion | 8 (12.9) | |||||||
| Colon/Esophagus | 2 (3.2) | |||||||||
| Melanoma | 1 (1.60) |
Abbreviation: BAT, brown adipose tissue; NOS, not otherwise specified.
aValues are expressed as No. (%)
In the current study, the most dominant distribution pattern of hypermetabolic BAT was a diffuse activation pattern in multiple anatomical sites in the neck, the mediastinal and paravertebral regions (21/62, 33.87%s). The highest level of metabolic activity in BAT depots represented by average SUVmax were recorded in the region of axilla (6.9, range: 1.2 - 25.5) followed by the neck (6.5, range; 1.7 - 17.3), and abdomen (5.52, range: 3.9 - 9.8). The anatomic and metabolic data of F-18 FDG detectable BAT depots are summarized in Table 4.
| Anatomical region | No. (%) | Average SUVmax (range) |
|---|---|---|
| Neck | 58 (93.54) | 6.5 (1.7 - 17.3) |
| Paravertebrae | 54 (87.09) | 4.74 (1.3 - 18.1) |
| Mediastinum | 38 (61.29) | 3.6 (1.4 - 10.7) |
| Axilla | 32 (51.61) | 6.91 (1.2 - 25.5) |
| Abdomen | 7 (11.29) | 5.52 (3.9 - 9.8) |
| Distribution pattern | ||
| Neck-mediastinum- paravertebrae | 21 (33.87) | |
| Neck-axillary-paravertebrae | 16 (25.8) | |
| Neck- paravertebrae- mediastinum- axilla | 11 (17.74) | |
| Neck- paravertebrae | 5 (8.06) | |
| Neck- paravertebrae- mediastinum- axilla-abdomen | 5 (8.06) | |
| Neck- mediastinum- axilla | 2 (3.22) | |
| Neck- axilla | 2 (3.22) |
Abbreviation: SUVmax, maximum standardized uptake values.
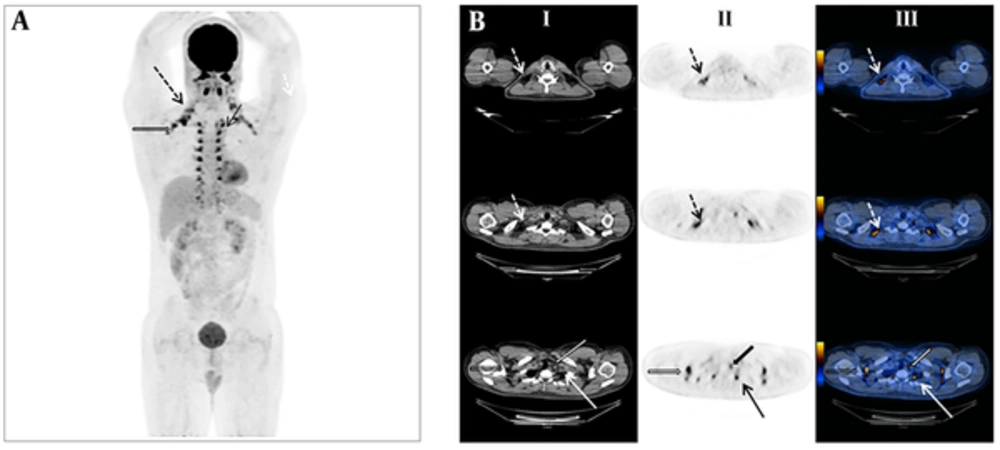
There was no statistically significant difference in the distribution pattern of active BAT regarding type (P = 0.135) as well as primary site of primary cancer (P value = 0.3). Figure 1 illustrates the most common cancer characteristics in the present report which demonstrated diffuse foci of BAT related F-18 FDG uptake in the neck, axillary, paravertebral and mediastinal region in a 46-year-old man with Hodgkin’s lymphoma who was referred for treatment response evaluation and proved to be in completed remission. The hottest spots are noted in axillary brown adipose tissue.
A, Anterior maximum intensity projection (MIP) demonstrated generalized 18-F fluorodeoxyglucose (FDG) uptake in brown adipose tissue located in the neck (dotted arrows), axillary (thick non-filled arrows), paravertebral (thin arrows) and mediastinal (thick filled arrows) regions in a 46-year-old man with Hodgkin’s lymphoma referred for treatment response evaluation. B, Axial non enhanced computed tomography (NECT) (column I), PET (column II) and fused positron emission tomography (PET)/CT (column III) images confirmed brown adipose tissue (BAT) related F-18 FDG uptake in the same patient with no evidence of metabolically active lymphadenopathy consistent with complete metabolic response to chemotherapy.
SUVmax demonstrated a weak inverse correlation with age (0.015, r = -0.32). The average SUVmax in patients < 40 years old was significantly higher than those who were older (8.24 vs. 4.33, U = 105, P value = 0.005). In addition, the average SUVmax was higher in patients with no evidence of active malignant lesion based on PET/CT images (8.98 vs. 5.81, P = 0.03) as well as those within the post-treatment period (9.5 vs. 5.1, P = 0.03). There was a significant correlation between the average SUVmax and the absence of active malignant lesion (0.03) as well as the recent history of treatment (0.011). In the patient group with a recent history of treatment, there was no significant difference in average SUV max between active (10/25, average SUVmax = 7.3) and non-active (15/25, average SUV max = 10.86) underlying malignancy (P value =0.14). However, in the absence of metabolically active cancer disease, the average SUVmax in patients with a recent history of treatment demonstrated to be significantly higher than in the patients who were referred for the indications other than evaluation of response to treatment (10.86 and 3.80, respectively, U = 14, P value = 0.040). A multiple regression test demonstrated age and recent history of treatment as independent predictors of the level of BAT metabolic activity, represented by SUVmax F (2.52) = 3.32, P = 0.044, R2 = 0.11, p value for age = 0.021, p value for recent treatment = 0.033. This test failed to reveal any significant correlation between average SUV max and gender, anthropometric characteristics, cancer type, reason for referrals, disease status and season based on univariate analysis. Comparison within each variable group including type of cancer, disease status and reason for referral revealed that though not statistically significant, the average SUVmax was higher in patients with primary diagnosis of Hodgkin’s disease (11.11 vs. 6.4), partial metabolic response (10.3 vs. 5.9) and treatment response evaluation (9.55 vs. 5.58), respectively.
5. Discussion
Brown adipose tissue as one of the main potential sources of false positive finding in F-18 FDG PET/CT is now recognized as a key player in regulating human metabolic profile. However, heat generating process modulated by BAT mitochondrial oxidative metabolism may increase cell vulnerability to cancer development via producing free radical oxidative and resultant oncogenic mutation (17) boosted by native adipocyte misbehaving (18) and accumulation of related metabolic pathway end products in tumor microenvironment; e.g. free fatty acid (4, 11, 19), though clinically-proven evidence is scarce.
Experimental and PET-based studies have provided strong evidence for breast cancer to have association with BAT activation even when compared to a sex and age-matched control group (14, 16). However, for other cancer types, the results are conflicting. While lymphoma was the most prevalent cancer in BAT bearing patients in the current study, it was confirmed as the lowest in another report. This disparity may at least be partly explained by the diversity of the disease stage in different studies. In the present study, the vast majority of patients with lymphoma underwent PET/CT scan in a 1- to 4-month interval period after the end of chemotherapy (77.27%). In addition, the level of BAT metabolic activity showed strong independent correlation with the recent history of treatment. These findings support a presumed confounding role of chemotherapeutic agent to increase BAT metabolic activity. The confounding effect of chemotherapeutic agents in BAT metabolic activity may provide an explanation for the lowest percentage of lung cancer in BAT bearing cohort most of whom were referred for initial staging without any recent history of treatment. The results of one study suggested that BAT depiction rate was higher in follow-up studies of pediatric patients with lymphoma when no evidence of active disease was present in comparison with the initial studies with no recent history of treatment and hence concluded that there may be an inverse relationship between PET positive cancer related findings and BAT visualization on F-18 FDG PET/CT (13). In contrast, another report conducted on adult patients with breast cancer aimed to assess the brown adipose tissue metabolic behavior during chemotherapy in consecutive F-18 FDG PET/CT studies failed to establish any correlation between biopsy-proven tumor response and detectable brown adipose tissue (14, 15). Such discrepancy may be explained by the potential confounding effect of chemotherapeutic agents. In the former study, the higher frequency of BAT visualization in follow-up studies most of which are expected to be performed in post treatment status may be attributable to the effect of chemotherapy rather than the absence of metabolically active cancer-related lesion since no significant association was proved between BAT recruitment and disease remission during chemotherapy in the latter study. It can be inferred that in addition to the presumed contributory role of activated brown adipocytes in cancer development and progression, BAT visualization in cancer patients may be the result of other cancer related extrinsic factors such as chemotherapeutic agents.
In line with the results of other studies, female gender, younger age, low/normal BMI and BF and autumn/winter seasonal pattern were more prevalent in BAT bearing patients (20-35). However, the level of BAT metabolic activity demonstrated significant correlation only with age and the recent history of treatment. It can be inferred that distinct pathways may be involved in BAT induction and regulation of the level of metabolic activity.
There were some major drawbacks in the present study. The current investigation focused on the correlation between the potential independent variables on the level of BAT metabolic activity. The direct effect of treatment as well as other cancer characteristics on BAT development in cancer patients requires a large-scale control study matched for age and sex as the proved influencing factors on BAT recruitment. In addition, some important baseline records including indoor and outdoor temperature, daylight duration and the details of chemotherapeutic regimens were not available. A relatively small sample size in each subgroup, cohort heterogeneity, lack of histological confirmation for brown adipose tissue and also lack of standard of reference for both negative and positive F-18 FDG PET/CT results should also be considered as additional major limitations in this report.
In conclusion, the present study provided evidence for age and chemotherapeutic agents to have correlation with the level of BAT metabolic activity, represented by average SUVmax as the most validated metabolic parameter of F-18 FDG PET/CT. Regarding the exclusive correlation of BAT metabolic activity with age and recent history of treatment, there is a suggestion for different pathways involved in BAT development and regulation of the level of metabolic activity.