1. Background
Pelvic floor disorder (PFD) constitutes a spectrum of various pathologies that account for a variety of clinical presentations and syndromes. Clinical complaints include urinary and anal incontinence, chronic pelvic pain, sexual dysfunction, constipation and urogenital prolapse (1, 2). They make a considerable decrease in the quality of life and involve up to 30% - 50% of middle-aged women (1-3). Pelvic structures could be divided into passive and active structures. Bones, supporting ligaments and fasciae constitute passive structures and pelvic floor muscles are active structures (4). Presence of the static and dynamic anatomical elements together reminds the complexity of the anatomy and pathophysiology of PFD. This makes the diagnosis of PFD challenging. In this regard, the first clinical assessment is clinical examination, but this assessment has challenges and pitfalls, is subjective and could not prepare data on all internal organs and structures of the pelvic floor (3). The recurrence rate of PFD symptoms after surgery is high. Up to 30% of patients with anterior compartment prolapse need another surgery (1). One of the reasons for such a high recurrence rate could be the incorrect or incomplete diagnosis before surgery. It has been reported that in many cases, the problem is complex and multicompartmental treatment approach is needed (1). On the other hand, a correct surgical plan depends on the clinical and paraclinical findings and we need a reliable scoring method to rate the severity of pathologies. Other paraclinical assessments have been introduced in this regard; previously defecogram and recently magnetic resonance imaging (MRI) could be mentioned in this regard. Defecogram is expensive and has some shortcomings. Regarding radiation to the pelvic area, it is invasive. In addition, findings could be confusing due to overlapping of the rectovaginal opacities. It could not yield any data on fasciae and pelvic floor muscles. MRI especially dynamic MRI is one of the most important methods that has been considered in the recent years as it is not invasive and could yield visualization of all soft tissues and pelvic compartments (1-18). Different MRI sequences and protocols could assess these structures. High spatial resolution static sequences are used for delineation of both passive and active structures, while detection of functional abnormalities requires fast imaging dynamic sequences in rest and straining (10).
2. Objectives
MRI could yield important information on these patients and the clinical exam is a subjective assessment that needs experience. Therefore in this paper, we are going to assess the agreement between clinical examination findings and MRI in scoring the Pelvic organ prolapse quantification (POP-Q) scaling.
3. Patients and Methods
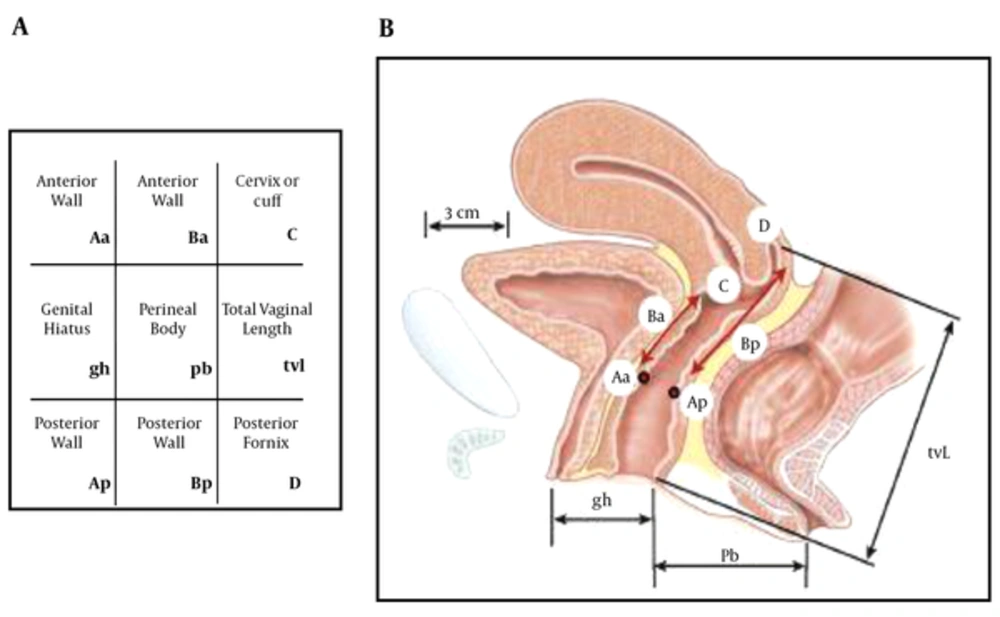
This was an experimental study of 61 randomly selected female patients. The patients were referred to PFD clinic in Imam Khomeini hospital from September 2013 to August 2014. Pelvic floor disorder was confirmed when the patient had prolapse-related symptoms such as urine incontinence, defecation problems and pelvic pain. Precise history and physical exam was elicited from all patients by an experienced gynecologist in the field of PFD, then a complete POP-Q exam was done for each patient. The radiologist and gynecologist were blinded regarding each other’s data. In this exam, urethral hypermobility, perineal descent, levator muscle contraction, prolapses in different compartments of pelvis (including anterior, posterior and apical compartments) and also the presence of entrocele and paravaginal prolapse were assessed (Figure 1 and Table 1).
| Stage | Description |
|---|---|
| Stage 0 | No prolapse is demonstrated. Points Aa , Ap, Ba, and Bp are all at -3 cm and either point C or D is between -tvl (total vaginal length) cm and - (tvl - 2) cm (i.e., the quantitation value for point C or D is ≤ - (tvl - 2) cm). |
| Stage I | The criteria for stage 0 are not met, but the most distal portion of the prolapse is > 1 cm above the level of the hymen (i.e. its quantitation value is < -1 cm). |
| Stage II | The most distal portion of the prolapse is ≤ 1 cm proximal to or distal to the plane of the hymen (i.e., its quantitation value is ≥ -1 cm but ≤ + 1 cm). |
| Stage Ill | The most distal portion of the prolapse is > 1 cm below the plane of the hymen but protrudes no further than 2 cm less than the total vaginal length in centimeters (i.e. its quantitation value is > + 1 cm but < +( tvl - 2) cm). |
| Stage IV | Essentially, complete eversion of the total length of the lower genital tract is demonstrated. The distal portion of the prolapse protrudes to at least (tvl - 2) cm (i.e., its quantitation value is ≥ + (tvl - 2) cm). In most instances, the leading edge of stage IV prolapse is the cervix or vaginal cuff scar. |
Stages of Pelvic Organ Prolapse
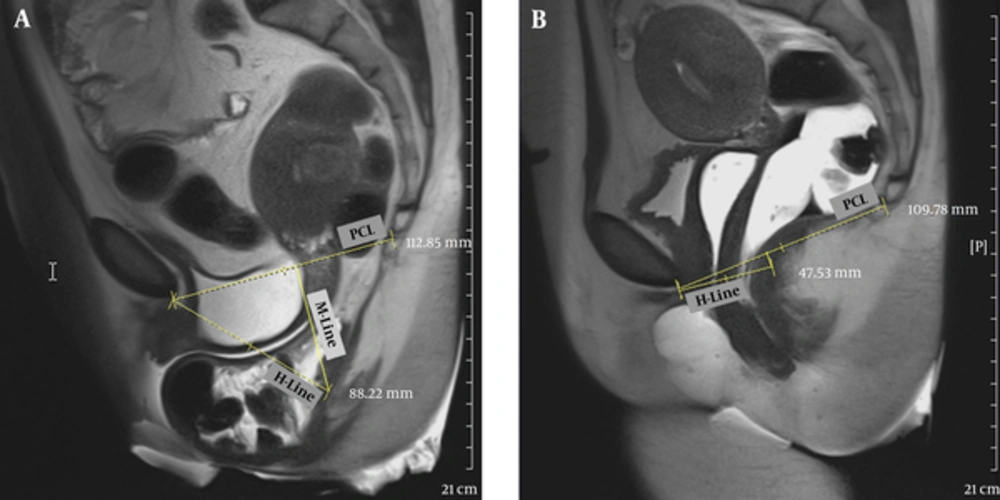
All patients underwent MR imaging thereafter. The applied magnetic imaging device was Siemens 3 Tesla Magnetom. In the process of magnetic resonance imaging preparation, all patients had to evacuate their bladder before imaging. Rectovaginal filling was performed respectively using nonsterile ultrasound gel to opacify the vagina (5 cc) and rectum (60 - 120 cc) before imaging. All patients were undergone imaging in supine position, using Coil Phase Array (16 channel body coiling). Static MR imaging in the supine position with T2-weighted fast spin-echo (T2 FSE) sequence in axial, and sagittal position, and dynamic MR imaging in midsagittal position, with T2 weighted half-Fourier acquisition single-shot turbo spin-echo (T2 HASTE) sequence in rest, during Valsalva maneuver, true FISP imaging during squeezing and at last defecogram images were obtained. Several lines were precisely drawn on sagittal MR images to assess and measure the extent of pelvic floor dysfunction at rest and during straining. The pubococcygeal line (PCL) joins the inferior border of the symphysis pubis to the last horizantally seen coccygeal joint in the midsagittal plane and it is the reference line for most measurements, H-line is the line drawn from the inferior border of the symphysis pubis to the posterior border of the anorectal junction. It is used to measure the anteroposterior width of the pelvic hiatus, which normally should not be more than 5 cm in its sagittal direction at rest or during defecation. M-line is traced between the anorectal junction and the PCL. It should not exceed 2 cm. Grading of prolapses in relation to PCL and H-Line are explained in Tables 2 and 3.
| Grade | Organ Location Relative to PCLa |
|---|---|
| 0 (no prolapse) | Above |
| 1 ( mild or small) | 0 - 3 cm below |
| 2 (moderate) | 3 - 6 cm below |
| 3 (severe or large) | ≥ 6 cm below |
Grading of Pelvic Organ Prolapse
| Grade | Organ Location Relative to H Linea |
|---|---|
| 0 (normal) | Above |
| 1 (mild or small) | 0 - 2 cm below |
| 2 (moderate) | 2 - 4 cm below |
| 3 (severe or large) | ≥ 4 cm below |
Grading of Pelvic Organ Prolapse
Furthermore, the data was graded from normal to severe, clinical findings were exactly noted and the results of MRI findings were compared with POP-Q examination for each patient. Cystocele is due to tearing or stretching of endopelvic fascia that causes bladder herniation on the anterior vaginal wall. At MR imaging, it is defined as a descent of the bladder base below the PCL. It may be associated with damage to the urethral suspension ligaments and urinary incontinence (19).
3.1. Statistical Analyses
We used SPSS ver. 18 for Windows (SPSS Inc. Released 2009. PASW Statistics for Windows, Version 18.0. Chicago: SPSS Inc.) for statistical analysis. Normality of data was assessed by Kolmogorov-Smirnov test. Difference of means between groups was determined by t-test. Agreement between different measurements in MRI and clinical exams were determined by Kappa coefficient of agreement. We interpreted the Kappa coefficient as excellent when they were 0.8 - 1, good when they were 0.6 - 0.8, intermediate when they were 0.4 - 06, and weak when they were 0.2 - 0.4. All P-values lower than 0.05 were considered as statistically significant.
4. Results
Totally 61 patients were assessed in this study. The mean age of the patients was 52.1 ± 15.1 (17-93).
Thirty-one patients had gone through menopause (59.6%), while 21 were fertile (40.4%). Among patients with the data on the delivery type, 34 out of 38 patients (89.5%) had normal vaginal delivery (NVD) and one patient reported both NVD and cesarean section (C/S). The mean delivery pregnancy number was 4.6 ± 2.5 and the mean delivery number was 4.5 ± 2.2. Stress incontinence was positive in 18 patients (29.5%) and urge incontinence was positive in 17 patients (27.9%). Constipation was seen in 20 patients (32.8%) and fecal splitting in 13 patients (21.3%). Other symptoms are mentioned in Table 4. Regarding physical exam findings, urethral hypermobility was seen in 55 patients (90.2%). Other signs have been mentioned in Table 4.
| Symptom | Subgroups | No (%) |
|---|---|---|
| Incontinence | Stress | 18 (29.5) |
| Urge | 17 (27.9) | |
| Mix | 1 (1.6) | |
| Fecal complaints | Constipation | 20 (32.8) |
| Splitting | 13 (21.3) | |
| Straining | 8 (13.1) | |
| Gas Incontinence | 6 (9.8) | |
| Sexual Activity | No | 20 (33.9) |
| Normal | 29 (49.2) | |
| Abnormal | 10 (16.9) | |
| Dyspareunia | 6 (9.8) | |
| Incontinence | 2 (2.3) | |
| Partner dissatisfaction | 3 (4.9) | |
| Sign | Subgroups | Abnormal or Positive |
| Urethral Hypermobility | 55 (90.2) | |
| Cough Test | 7 (11.5) | |
| Vaginal Atrophy | 36 (59) | |
| Perineal Defect | 47 (77) | |
| Perineal Descent | 48 (78.8) | |
| Levator Contraction Tone | Good | 19 (31.2) |
| Moderate | 30 (49.2) | |
| Weak | 12 (19.7) | |
| Anal Exam | 7 (11.5) | |
| Anterior Prolapse | No | 9 (14.8) |
| Stage I | 5 (8.2) | |
| Stage II | 28 (45.9) | |
| Stage III | 17 (27.9) | |
| Stage IV | 2 (3.3) |
Distribution of Different Symptoms and Signs Among Patients
In MRI assessment, we measured important different lines including bladder neck descent [regarding pubococcygeal (PCL) and H-line] and urethral descent [regarding PCL and H-line] (Table 5).
| Variable | Reference Line | Mean ± SD, mm | Range, mm |
|---|---|---|---|
| Bladder Neck Descent | PCL | 24.2 ± 32.1 | -30 - 110 |
| Bladder Neck Descent | H | 7.3 ± 25.4 | -36 - 84 |
| Urethral Descent | PCL | 12.4 ± 24.1 | -30 - 120 |
| Urethral Descent | H | 0.8 ± 16.2 | -34 - 65 |
Mean Distance of Different Anterior Pelvic Organ Descents and/or Prolapses Considering Pubococcygeal and H lines in Magnetic Resonance Imaging
For assessment of agreement between POP-Q staging by physical exam and MRI findings in anterior pelvic compartment, we categorized the MRI data based on the reference methods to different classes of normal and mild, moderate and severe prolapses. Then we cross-tabulated the data of POP-Q versus the MRI findings calculated based on the mentioned method. For this purpose, we considered different classes in four situations. In the first step, we considered the POP-Q and MRI classes in four detailed subclasses. In POP-Q, we had normal and stages I-IV. We mixed stages III and IV together. Thus, we have four groups of normal and stages I, II, and III and IV together. In MRI, we had four subgroups of normal (equal to normal subgroup in POP-Q), mild prolapse (equal to stage I in POP-Q), moderate prolapse (equal to stage II in POP-Q) and severe prolapse (equal to stages III and IV in POP-Q). We calculated the Kappa based on four subgroups. In the next steps, we considered different cutoff points for classification of patients in two groups. First, cutoff points including normal versus abnormal patients. Second, cutoff points classified normal and mild prolapses together versus moderate and severe prolapses together. Third, cutoff points classified normal to moderate subgroups together versus severe patients. Then we calculated Kappa coefficients in all corresponding situations between POP-Q and MRI (Tables 6 - 8).
| MRI Staging Based on PCL | ||||||
|---|---|---|---|---|---|---|
| Normal | Mild | Moderate | Severe | Kappa Coefficient | ||
| POP-Q | Normal | 5 | 4 | 0 | 0 | 0.18 |
| Grade I | 2 | 2 | 1 | 0 | ||
| Grade II | 7 | 12 | 8 | 0 | ||
| Grade III and IV | 1 | 3 | 8 | 7 | ||
| MRI Staging Based on H-Line | ||||||
| POP-Q | Normal | 5 | 3 | 0 | 0 | 0.05 |
| Grade I | 5 | 0 | 0 | 0 | ||
| Grade II | 13 | 12 | 2 | 0 | ||
| Grade III and IV | 5 | 3 | 5 | 6 | ||
Agreement of POP-Q and MRI for Bladder Neck Considering Pubococcygeal Line and H-Line
| MRI Staging Based on PCL | ||||||
|---|---|---|---|---|---|---|
| Normal | Mild | Moderate | Severe | Kappa Coefficient | ||
| POP-Q | Normal | 4 | 4 | 0 | 0 | 0.06 |
| Grade I | 2 | 2 | 1 | 0 | ||
| Grade II | 7 | 17 | 3 | 0 | ||
| Grade III and IV | 2 | 14 | 1 | 2 | ||
| MRI Staging Based on H-Line | ||||||
| POP-Q | Normal | 5 | 3 | 0 | 0 | -0.04 |
| Grade I | 5 | 0 | 0 | 0 | ||
| Grade II | 16 | 11 | 0 | 0 | ||
| Grade III and IV | 6 | 9 | 3 | 1 | ||
Agreement of POP-Q and MRI for Urethra Considering Pubococcygeal Line and H-Line
| Anatomical Region | Cutoff Point | Reference Line | Both MRI and POP-Q Positive | Both MRI and POP-Q Negative | MRI Negative and POP-Q Positive | MRI Positive and POP-Q Negative | Kappa (95% Confidence Interval) | Concordance Rate, No.(%) |
|---|---|---|---|---|---|---|---|---|
| Bladder Neck | Normal vs. Abnormal | PC Line | 41 (68) | 5 (8) | 10 (17) | 4 (7) | 0.28 (0.004 - 0.56) | 46 (77) |
| (Normal to Moderate) vs. (Severe) | PC Line | 7 (12) | 41 (68) | 12 (20) | 0 | 0.44 (0.21 - 0.68) | 48 (80) | |
| Urethra | Normal vs. Abnormal | PC Line | 40 (68) | 4 (7) | 11 (19) | 4 (7) | 0.21 (0 - 0.48) | 44 (75) |
| (Normal to Moderate) vs. (Severe) | PC Line | 2 (3) | 40 (68) | 17 (29) | 0 | 0.14 (0 - 0.31) | 42 (71) | |
| Bladder Neck | Normal vs. Abnormal | H Line | 28 (47) | 6 (10) | 23 (38) | 3 (5) | 0.09 (0 - 0.3) | 34 (57) |
| (Normal to Moderate) vs. (Severe) | H Line | 6 (10) | 40 (68) | 13 (22) | 0 | 0.39 (0.15 - 0.62) | 46 (78) | |
| Urethra | Normal vs. Abnormal | H Line | 24 (41) | 5 (8) | 27 (46) | 3 (5) | 0.04 (0 - 0.2) | 29 (49) |
| (Normal to Moderate) vs. (Severe) | H Line | 1 (2) | 40 (68) | 18 (31) | 0 | 0.07 (0 - 0.2) | 41 (69) |
Agreement of POP-Q and MRI Findings for Anterior Pelvic Anatomical Organs Considering Pubococcygeal and H Lines in Differentiating Normal Subjects and Severe Prolapsesa
5. Discussion
Pelvic floor dysfunction (PFDs) refers to a wide range of issues that occur when muscles of the pelvic floor are weak, tight, or there is an impairment of the sacroiliac joint, lower back, coccyx, or hip joints. PFDs affect about 50% of women past middle age. Symptoms include pelvic pain, urinary and bowel incontinence and pelvic organ protrusions, dyspareunia, incontinence, and incomplete emptying. Nowadays, there are novel diagnostic tools and therapies, proposed for pelvic floor weakness and organ prolapses. POP-Q examination and complementary MRI are two methods of diagnosis (1-3).
The POP-Q method is the current standard for assessment of POP-Q. However, MRI-based staging using the pubococcygeal line (PCL), midpubic line (MPL), perineal line, and H line as references, could be used as a complementary diagnostic method for prolapse staging. The high soft tissue resolution capability of MRI, provides tissue characterization and in detail information about pelvic pathologies, organ prolapses, or even organ supporting structure failures. It is also capable of multi-planar imaging capabilities. The main purpose of the present study was to correlate pelvic organ prolapse on dynamic pelvic MRI in the supine position with pelvic organ prolapse using POP-Q exam and propose MR imaging as a complementary method of POP-Q diagnosis hoping to decrease the recurrence rate.
Our study sheds light on the fact that MR imaging can increase diagnostic power of detecting subtle lateral wall defects in anterior compartments.
The possible explanation is that there is considerable variability in the technical performance of the specific measures in POP-Q staging and it can miss some minor clinical problems.
As noted earlier, we considered the pubo-coccygeal line (PCL) and H line as reference lines in the current study. Comparing dynamic MRI findings and POP-Q exam findings showed acceptable to a good agreement rate (49% - 80%) (corresponding to a weak to moderate agreement) between MRI and physical examination regarding different anterior anatomical organs (bladder neck and urethra). These findings were yielded when we considered different cutoff points. The first cutoff point classified normal versus abnormal groups with the goal of detecting the main diagnosis of PFD and second cutoff point classified normal to moderate prolapses versus severe prolapses for deciding on the indication of surgical plan.
Considering PCL for differentiating patients with any bladder neck prolapse (means setting cut off point in normal vs. abnormal point), 76% of patients had similar findings in MRI and POP-Q. These included 68% of patients who showed positive findings in both MRI and POP-Q and 8% who showed negative findings in both methods. In 7% of the patients, MRI also detected the missed pathologies and prolapses by POP-Q examination. This means a totally 76% agreement rate between MRI and manual exam.
In this situation, when we considered differentiating cutoff point higher (normal to moderate vs. severe), 80% had similar findings in MRI and POP-Q. These included 12% who showed positive findings in both methods and 68% who showed negative findings in both methods. This means a 80% agreement rate between MRI and POP-Q exam. POP-Q examination missed none of the prolapses.
Considering PCL for differentiating patients with any urethral prolapses (means setting cut off point in normal vs. abnormal point), 75% had similar findings in MRI and POP-Q. These included 68% of patients who showed positive findings in both MRI and POP-Q and 7% who showed negative findings in both methods. This means 75% total agreement of findings between MRI and POP-Q. In 7% of the patients, MRI detected pathologies that POP-Q examination did not detect them.
In this situation, when we considered a differentiating cutoff point higher (normal to moderate vs. severe), 71% had similar findings in MRI and POP-Q. These included 3% who showed positive findings in both methods and 68% who showed negative findings in both methods. This means a 71% agreement rate between MRI and POP-Q exam. POP-Q examination missed none of the prolapses.
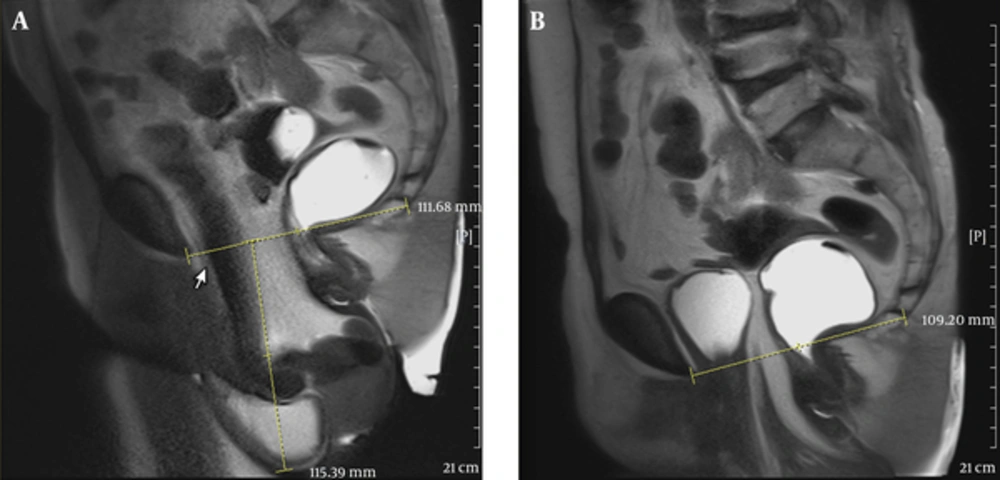
In the anterior compartment, higher agreement rates when we considered PCL as reference line compared to H-line is due to this fact that PCL is a fixed anatomical
line, while H-line is movable and can easily change position whenever anorectal angle changes (Figure 2).
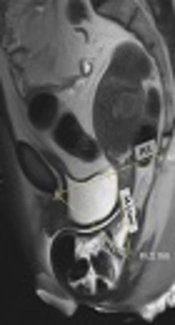
A 44-year-old female with chief complaints of obstructed defecation, frequency and mixed urinary incontinence (urge and stress). A, Midsagittal T2 HASTE during rest shows pubococcygeal line (PCL) between the inferior margin of symphysis pubis and last horizontally oriented coccygeal line. H-line is between the inferior margin of symphysis pubis and posterior aspect of anorectal angle. M-line is between PCL and H-Line; B, Midsagittal T2 HASTE during straining shows small cystocele, moderate apical descent and large rectal descent with anterior rectocele.
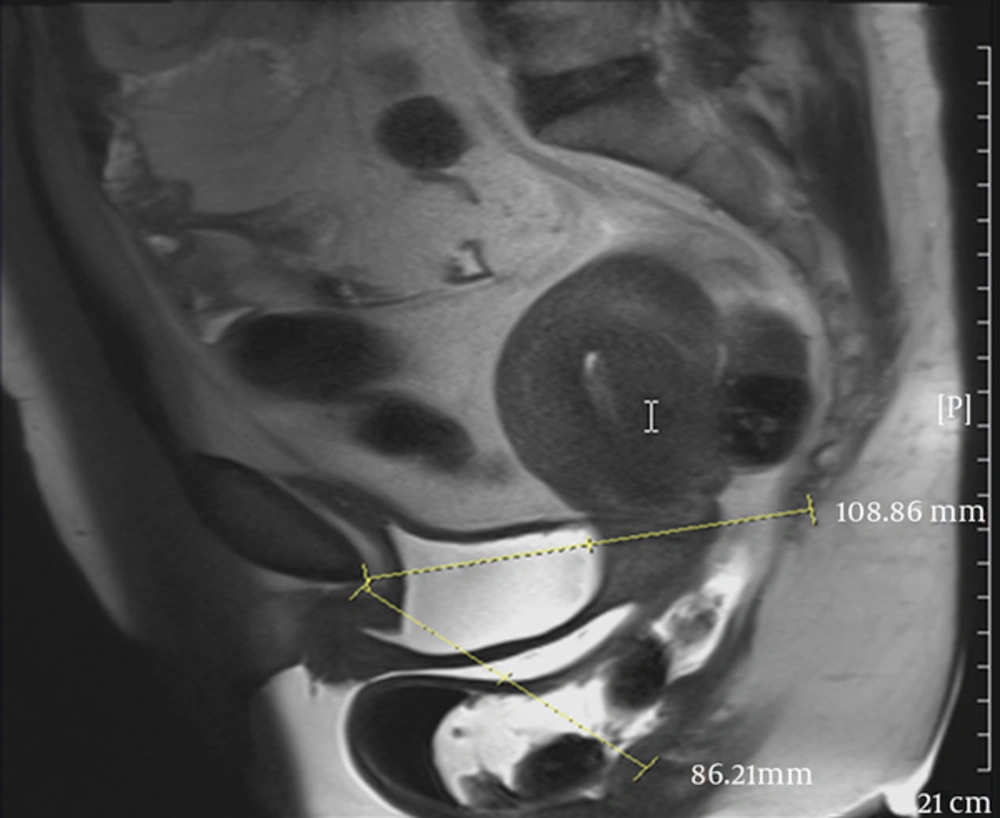
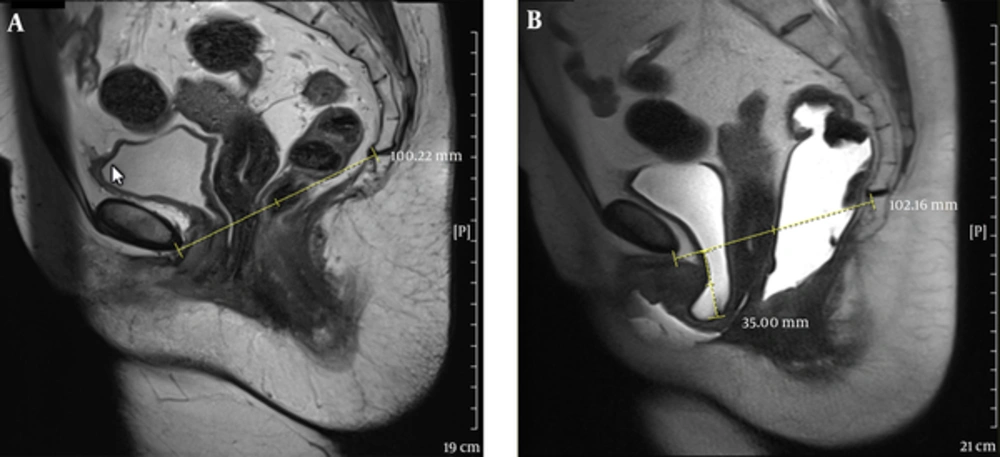
The disagreement of MRI and POP-Q in the anterior compartment, especially in some anatomical site defects, could be due to capability of MRI in differentiation of an isolated cystocele from cystourethrocele possible (Figures 3 and 4).
A 50-year-old female with dyspareunia. A, Midsagittal T2 HASTE during rest shows the bladder neck at the level of pubococcygeal line (PCL). B, Midsagittal T2 HASTE during straining shows multi-compartment defect including large cystocele and urethral hypermobility, urethral kink with resultant bladder outlet obstruction and trabeculated bladder, large apical descent and rectal descent.
Kinking of the urethrovesical junction may be due to prolapse of the bladder base, which is a potential cause of urinary retention. This condition could be associated with or mask the symptoms of incontinence and can probably lead to urinary stasis and infections (20).
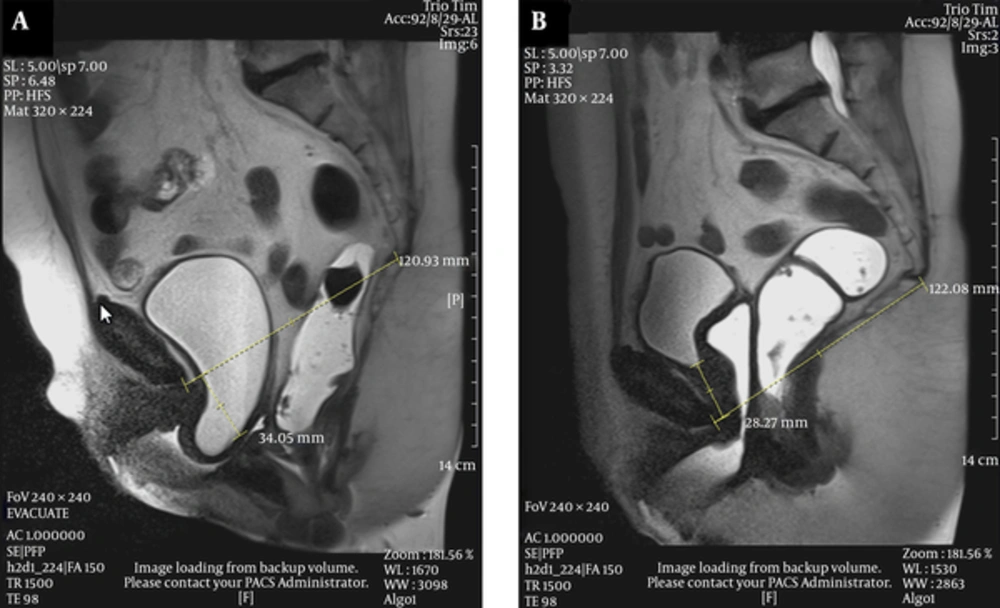
El-Gharib et al. reported that on functional MRI, considering anterior compartment, the best correlation of findings with POP-Q clinical results was obtained using PCL as the main reference line (21). They also found out that dynamic MRI is really helpful in defining nature, degree and quantitative measurements of visceral prolapses, while the solely use of POP-Q may be inadequate (21) (Figures 5 - 7).
A 67-year-old female with prior history of hysterectomy came with urge urinary incontinence. A, Midsagittal T2 HASTE during rest shows normal position of the bladder neck, vaginal apex and anorectal junction. B, Midsagittal T2 HASTE during straining shows moderate cystocele and urethral hypermobility, large apical prolapse and large rectal descent.
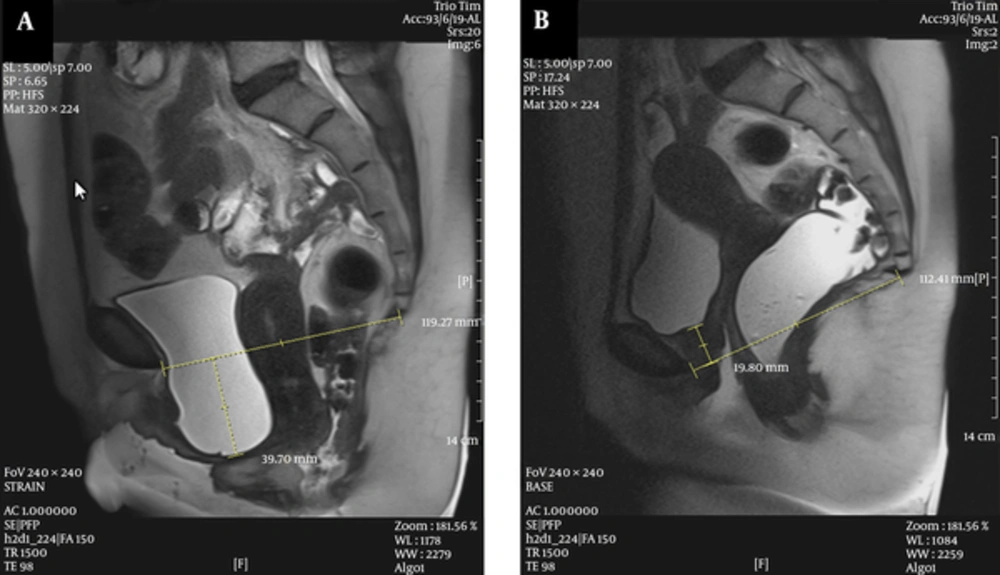
A 48-year-old female with perineal mass protrusion. A, Midsagittal T2 HASTE during rest shows the normal position of the bladder neck, vaginal apex, and mild low anorectal junction; B, Midsagittal T2 HASTE during straining shows large cystocele and urethral hypermobility with kinking and resultant bladder neck obstruction, and trabeculation, large apical prolapse, anterior rectocele, and rectal descent.
A 54-year-old female with bladder outlet obstruction and perineal mass protrusion. A, Midsagittal T2 HASTE during rest shows the bladder neck at the level of pubococcygeal line (PCL); B, Midsagittal T2 HASTE during straining shows large cystocele, severe apical descent and large enterocele containing illeal loops and mesenteric fat.
The results of our study are in complete agreement with those of a study conducted by Inas Azab et al. Including 40 cases in Inas study, they found 82.5% similarity of findings between MRI and POP-Q examination in the anterior compartment (3, 4). Using MR imaging, they detected only 10% of missed prolapse cases, which were not detected by POP-Q examination. They also reported 7.5% of MRI missed diagnoses in comparison to POP-Q in the anterior compartment (3, 4).
Lienemann et al. (22) and Gousse et al. (23) have also found good correlation of MR imaging and POP-Q examination in the anterior compartment.
Gupta et al. (24) found a poor correlation of MR imaging and POP-Q findings in the anterior compartment. This may be due to the different MRI staging system (HMO grading system).
Furthermore, we have not found any discrepancy of findings for those of Farouk El Sayed or Cimsit et al. studies (4, 5). They also reported good agreement of findings for MR imaging and physical examination in the anterior, middle and posterior compartments.
According to a study carried out by Gousse et al. including female patients with symptomatic pelvic prolapses, and considering cystocele, POP-Q examination was more than or equally sensitive to MR imaging. However, MRI was more sensitive for detecting entrocele and other detected pathologies that may have been missed on physical examination (23).
Singh et al. have also reported a good correlation of POP-Q with MR imaging for staging pelvic prolapses. They have also reported that MRI can clearly diagnose prolapses in the pouch of Douglas (25).
One of the limitations of this study was that we did not assess intrarater reliability for radiologist and gynecologist assessment. Interrater reliability is not proposed as the assessments were done by one radiologist and one gynecologist.
However, the gynecologist was an expert fellowship candidate of pelvic floor sub-speciality and her examinations were reliable. Also, as the radiologist was an MRI fellowship graduated attending member, her assessment was also reliable.
As the final word, we try to emphasize the good correlation between dynamic MR grading of pelvic organ prolapses and POP-Q clinical examination, which can affect the surgical decision. We also recommend MR imaging as a complementary method of study along with POP-Q in detecting pelvic organ prolapse and pathologies in clinical diagnostic approaches.