1. Introduction
Ovotesticular disorder of sex development (DSD) is a rare form of ambiguous genitalia. It is seen in approximately 3% - 10% of total DSDs (1, 2). It is defined as a congenital anomaly with the presence of both ovarian tissue and testicular elements in the same individual. The karyotype may be 46 XX, 46 XY, or a mosaic of 46 XX / 46XY (3, 4). The most common type is 46, XX and female sexual development. Internal and external genitalia of fetuses are indistinguishable until the sixth week of gestation. Gonads develop from the gonadal ridge. Germ cell development begins in the gonadal ridge during the sixth week of gestation. During this bipotential stage, various factors such as translocation of gene and hormonal abnormalities are involved in abnormal sex determination and differentiation. Interruption at any level can result in ambiguous genitalia (5, 6).
The magnetic resonance imaging (MRI) findings of a male patient who had ovotesticular DSD with mosaic chromosomal pattern and associated with unilateral seminal vesicle agenesis, hypospadias, presence of both ovary and testis and malignancies in both gonads are presented in this case.
2. Case Report
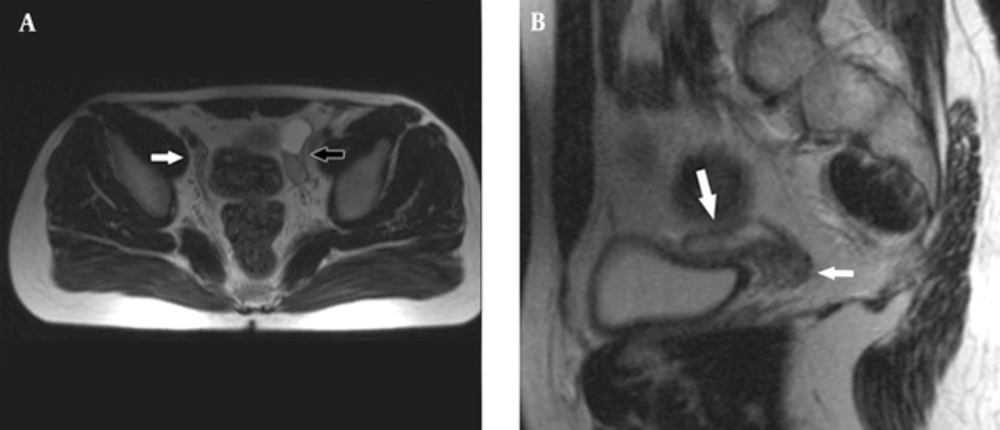
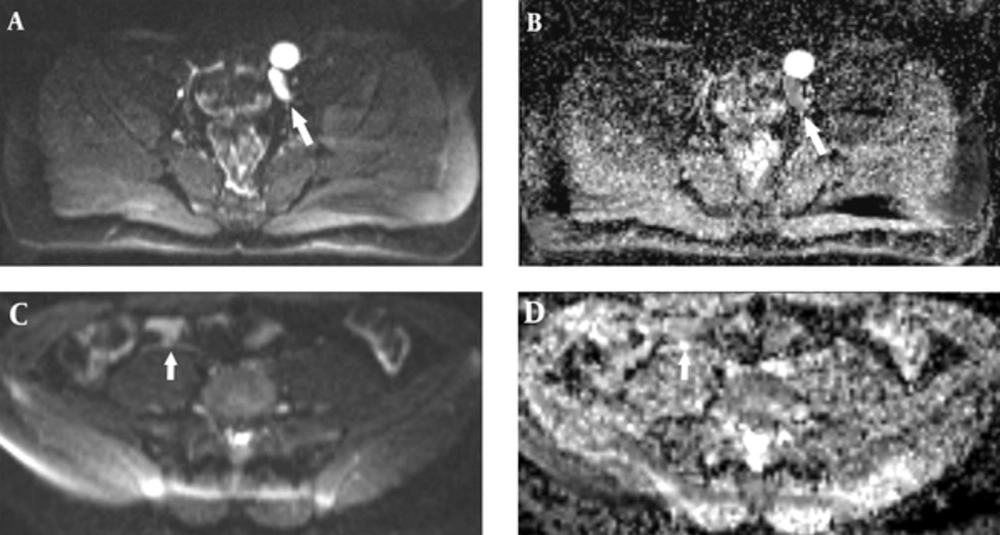
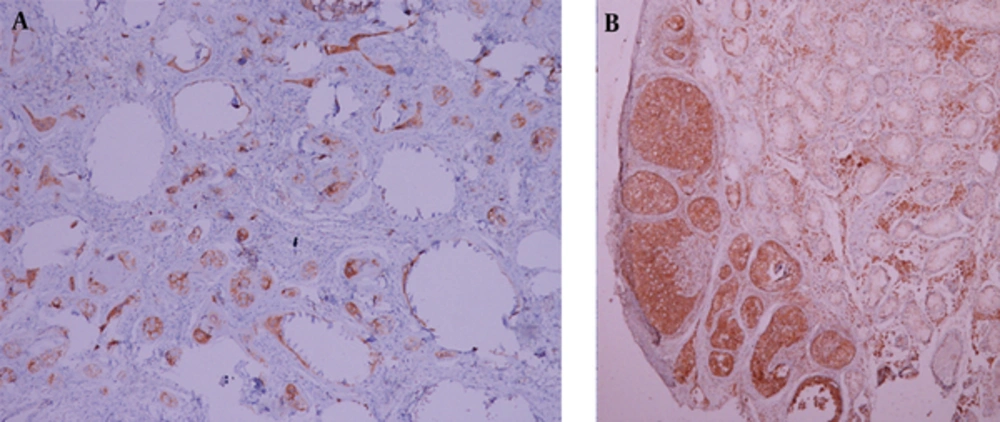
An 18-year-old male patient was referred to the hospital for nonpalpable testes and urination abnormalities. Penoscrotal hypospadias with nonpalpable testes were found on physical examination, The laboratory tests such as testosterone, luteinizing hormone (LH), lactate dehydrogenase (LDH), alpha fetoprotein (AFP), and beta human chorionic gonadotropin (ßHCG) levels were normal. On the other hand, there was a three-fold increase (36.38 mIU/ml) in the follicle stimulating hormone (FSH) level. Karyotype analysis was reported as 80% 46 XY and 20% 45X chromosomal pattern. Contrast-enhanced abdominal MRI and diffusion weighted imaging were performed to demonstrate associated anomalies. There was a left undescended testis with heterogeneous signal near the bladder. Epididymal cyst was found as a cystic lesion associated with left undescended testis. A linear structure like fallopian tube and a heterogeneous nodular lesion like ovary were seen on the right side on T2 weighted images (Figure 1). The right side gonad was thought to be an ovary on T2 sequences because of configuration and location on the distal end of the fallopian tube. The right seminal vesicle was normal, but there was no left seminal vesicle. Prostate and prostatic urethra were normally seen, but penile urethra was not visualized. Müllerian duct remnant as a linear cystic lesion was shown between the prostate and rectum. The fusion of müllerian duct remnant and prostatic urethra opened in the skin at the level of perineum. The restriction of diffusion which indicates increased cellularity was seen in both the right ovary and left testis (Figure 2). Diagnostic laparoscopy, hysterosalpingo-oophorectomy and abdominal orchiectomy were performed because of high suspicion of malignancy. Histopathologically, gonadoblastomas (in situ malignant germ cell tumors) were found in both the right ovary and left testis (Figure 3).
Fallopian tube (white arrow) on the right side of the bladder and testis with epididymal cyst (black arrow) on the left side of the bladder are shown on axial T2 weighted image (A) and fallopian tube (large white arrow) and right ovary (small white arrow) on the distal end of the fallopian tube on sagittal T2 weighted image (B).
3. Discussion
Ovotesticular DSD is a congenital anomaly characterized by the presence of both ovary and testis tissues (3,4). Genotypic sex is determined by chromosomes. For phenotypic sex development, these chromosomes activate some pathways and hormones. In the presence of sex-determining Y gene (SRY gene), differentiation into male phenotype begins. Absence or inactivation of SRY gene causes the development of the female phenotype (6). Fetus has both Mullerian and Wolffian ducts until the sixth week of gestation. In early gestational period (< 7 weeks), embryological developmental defect influences both Wolffian and Müllerian ducts (7). Testicular testosterone production promotes the growth of Wolffian duct. Anti-Mullerian Hormone (AMH) is secreted by sertoli cells. AMH is responsible for regression of Mullerian duct between 8 and 10 weeks of gestation. Any abnormality during gonadal development and differentiation of male or female phenotype can result in DSD. Either of the Mullerian duct remnants such as the uterus, fallopian tube, and cervix is also seen in patients with ovotesticular DSD (7).
In the presence of the penis, hypospadias is commonly seen. If testis is present, it is usually undescended. Due to undescended testes, infertility is a common problem but ovulation or spermatogenesis can occur. In delayed diagnosis of ovotesticular DSD, there is also a high risk of malignancy in the ectopic gonads (4, 5). Therefore, surgery is necessary in the treatment of ovotesticular DSD. The imaging modalities can be used for pre-operative evaluation of anatomy. Ultrasound (US), computed tomography (CT) or MRI can be performed. Thawait et al. (8) recommended MRI for evaluation of pelvic structures and masses. MRI multiplanar investigation with high contrast resolution provides excellent soft tissue characterization. The other advantage of MRI is lack of radiation exposure.
In conclusion, our case is very rare. In our case, in addition to the presence of the right ovary and left testis, ovotesticular DSD was associated with unilateral agenesis of the seminal vesicle, hypospadias, and also malignant tumors in the gonads. Pre-operative MRI is an effective modality for detecting the localization of ectopic testes and associated anomalies. MRI can be helpful for planning operation and demonstration of detailed anatomy.