1. Introduction
Interrupted aortic arch (IAA) is a rare condition that is defined as a lack of luminal continuity between the ascending and descending portions of the Aorta (1). There are three main types of IAA (A, B, and C), which were defined in 1959 according to the anatomical site of the aortic interruption (2). Type B is the most common type (comprising approximately two thirds of all cases) and the defect occurs between the left common carotid and left subclavian arteries.
Type A is the second most common type and makes up approximately one third of IAA cases. This type is an interruption of the aortic arch just distal to the left subclavian artery. Type C is a very rare type, occurring in less than 5% of IAA cases. In this type, the defect is the most proximal form, occurring between the innominate and left common carotid arteries (3).
In a study, 95 cases of IAA were reviewed by Schreiber et al. among which 84% were classified as type B, 13% as type A, and only 3% as type C (4).
Based on the status of the subclavian artery, each type could be divided into 3 subtypes:
- Normal subclavian artery (sub-type 1)
- Aberrant subclavian artery (sub-type 2)
- Isolated subclavian artery that arises from the ductus arteriosus (sub-type 3)
Right-sided aortic arch is such a rare condition that the prevalence in autopsy and radiographic studies is approximately 0.1% and it is even less common in the setting of normal cardiac situs and almost always has conjunction with other congenital cardiovascular anomalies.
Tetralogy of Fallot and truncus arteriosus have the most common association with right-sided aortic arch. In contrast to the left aortic arch, association of the right-sided aortic arch with aortic arch interruption is extremely rare. Moreover, all reported cases are type B according to a mirror image of the left-sided arch classification (5).
2. Case Presentation
A 4- year old boy with a history of poor feeding, dyspnea and irritability while feeding that started since infancy was referred to our center.
Long term examination of his disorder was started from the age of one month by echocardiography. Echocardiographic findings revealed severe pulmonary hypertension and large ventricular septal defect (VSD), and due to the severity of pulmonary stenosis (PS), he underwent VSD repair and pulmonary artery (PA) banding operation. However, after those interventions, clinical symptoms became more severe. Then, at the age of 2, for reaching a more definitive diagnosis of the underlying congenital heart disease, catheteric angiographic studies were performed and right sided aortic arch (RSAA), increased right ventricular (RV) and PA pressures were noticed in these studies.
Later, the patient was referred to our radiographic center to clearly delineate his cardiac function and the complex anatomy of his aortic arch. For this purpose, the patient underwent CT angiography with multislice spiral thin section scans plus maximum intensity projection (MIP), multiplanar reconstruction (MPR), and volume rendering reconstruction techniques.
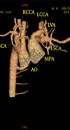
CT angiography findings revealed normal origination of the aorta, but the aortic arch was right sided and the left subclavian artery (SCA) and left common carotid artery (CCA) were isolated from the aortic arch connecting with the main pulmonary artery by a dilated and short trunk, probably patent ductus arteriosus (PDA); while right CCA and right SCA were divided from the aortic arch. The aortic arch was individually continued by the right-sided descending aorta, and the left SCA and left CCA were disrupted. Multiple dilated paravertebral and intercostal collaterals were also present (Figures 1 - 3).
Type C interrupted aortic arch (IAA) with right-sided aortic arch and double superior vena cava (SVC). Spiral CT angiography with volume rendering techniques. A and B, Coronal and axial front cut. C, Anterior view. D, Posterior view. E and F, With rotation. Vascular structures are pointed by arrows. (RV, right ventricle; RA, right atrium; RSVC, right superior vena cava; LSVC, left superior vena cava; AO, aorta; IVC, inferior venacava; MPA, main pulmonary artery; RPA, right pulmonary artery; LPA, left pulmonary artery; LCCA, left common carotid artery; RCCA, right common carotid artery; RSCA, right subclavian artery; LSCA, left subclavian artery; IAA, interrupted aortic arch; RSAA, right sided aortic arch.)
Type C interrupted aortic arch (IAA) with right-sided aortic arch and double superior vena cava (SVC). A - D, Spiral CT angiography with MIP reconstruction techniques. (RV, right ventricle; RA, right atrium; RSVC, right superior vena cava; LSVC, left superior vena cava; AO, aorta; MPA, main pulmonary artery; RPA, right pulmonary artery; LCCA, left common carotid artery; RCCA, right common carotid artery; IAA, interrupted aortic arch; MIP, maximum intensity projection.)
A, Aortic arches and dorsal aorta before transformation into the definitive vascular pattern. B, Aortic arches and dorsal aorta after the transformation. Broken lines: obliterated components. C, The great arteries in the adult. Figure 13.37 of 12th ed. Langman’s Medical embryology 19 ed. (6).
Development of the left ventricle (LV), left atrium (LA) and pulmonary veins (PVs) was normal and no abnormal connection was seen between the cardiac chambers. The connection of the right atrium (RA) with the RV was present. The development of the right ventricle outflow tract (RVOT) was also normal. A supravalvular PA band was noticed. Dilatation of the main pulmonary artery (MPA), left pulmonary artery (LPA) and right pulmonary artery (RPA) complexes was observed.
Right brachiocephalic vein (RBCV) was continued by the right superior vena cava (RSVC) and then drained into the right atrium (RA). Left brachiocephalic vein (LBCV) was continued by the left superior vena cava (LSVC) and then drained into the coronary sinus. RA and the coronary sinus were dilated. The connecting innominate vein was absent (Figures 1 and 2), but the development of the inferior vena cava (IVC) and azygos vein was normal. The visceral and bronchial situs solitus and left cardiac axis were observed. The main bronchus of the right upper lobe was divided from the trachea before the carina, as an independent branch. The carina was stenotic due to the pressure of the neighboring vascular structures.
3. Discussion
IAA is a rare condition with the prevalence of 3 - 20 in 1,000,000 live births. Among the three types of IAA, type C is the rarest one (3%). On the other hand, right sided aortic arch is a rare condition too, therefore, association of these two conditions would be extremely rare (7).
Factually, in contrast to the left aortic arch, the association of RSAA with IAA is very rare and all previously reported cases have been type B according to a mirror image of the left sided arch classification (5).
In a study by McElhinney and assistants who reviewed 16 cases of association of RSAA and IAA, all of the cases were of type-B interruption and none of them was similar to our case. According to the reasons that will be discussed below, we suppose that the type of aorta interruption in our case could be type-C.
From an embryologic viewpoint, IAA is an obstruction in some sites of the fetal arterial system that we expect to be normally open. During fetal life, the arterial system is formed by arterial arches (schematic Figure 3). Branchial arches develop during the fourth and fifth week of fetal life, and reach the aortic arches that are originated from the aortic sac. These arches are 6 pairs; the third pair of arches constitutes common carotid arteries and the first part of internal carotids.
The fourth pair of aortic arches remain bilaterally. On the left side, this arch constructs a part of aorta that is placed between the left CCA and left SCA and forms the most proximal part of the right SCA on the right side. The distal part is originated from a part of right dorsal aorta and the seventh intersegmental arteries.
The sixth pair of arches, the pulmonary arches, cuts its connection with the dorsal aorta on the right side, but this connection remains intact on the left side and creates ductus arteriosus.
The dorsal aortas disappear between the inlet of the third and fourth arches and form the carotid ducts. Also, a part of the right dorsal aorta that is placed between the origin of the seventh intersegmental artery and the left dorsal aorta connection, disappears normally and that is the reason why the left sided aortic arch appears at this stage (8).
With this background, one could infer that type A and type B IAA can be due to an obliteration of a part of the fourth aortic arch and dorsal aorta, while C type can be due to an obstruction of a part of the ascending aorta before the origination of the fourth arch, indeed.
In our case, the aortic arch is right sided which means the site of obliteration is between the seventh intersegmental artery origin and the left dorsal aorta instead of the right dorsal aorta, and as a result, right sided aortic arch is formed. In this case, another obstruction also occurred in the proximal part of the left fourth aortic arch, before separation of the common carotid and left SCA that may be equivalent with type C interruption. A similar case was reported in 1979 which was entitled “right sided aortic arch with isolation of innominate artery” (9).
With embryologic considerations, this case could be considered as a type C interruption and we will explain our reasons in the below paragraphs. As previously mentioned, IAA has three types, A, B, and C, and based on the location of the right subclavian artery, each of them is divided into three subtypes.
In subtype 1, the right subclavian artery (RSA) originates normally. In subtype 2, RSA is originated from left sided aortic arch as an aberrant artery, and in subtype 3, it is separated from the right PDA as an isolated artery. The reason of these events, can be explained by embryology and in the following, differences of subtypes of type C IAA and the reason why we believe that the case presented in this article may be a new subtype, will be explained.
As we know, the right subclavian artery is the result of development of the seventh intersegmental artery, which originates from dorsal aorta just distal to the origin of the sixth arch. It is along with the right fourth arch after obliteration of the right fifth arch and the posterior part of the right sixth arch and therefore, the subclavian artery and right common carotid artery are separated from the brachiocephalic common trunk, which is the remnant of the right ascending aorta branched from the aortic sac. As mentioned previously, type C IAA is the result of obliteration of a segment of the left ascending aorta branched from the aortic sac just before separation of the left fourth aortic arch.
For better understanding, in the following schematic Figures 4, the sites of obliterations occurred in type C IAA are shown and Figure 4D reveals normal obliteration sites.
Sites of obliteration that occur in type C interrupted aortic arch (IAA). A, Sites of obliteration that occur in subtype C1 IAA. Arrow A3 points to the posterior site of the sixth arch, which is equivalent with right patent ductus arteriosus (PDA) that is obliterated normally. Obliteration of the left branch of the ascending aorta originated from the aortic sac before separation of the fourth arch, which is equivalent with type C IAA (Arrow A1). Arrow (A2) points to obliteration of distal of right dorsal aorta distal to the left seventh intersegmental artery, which forms on the future right subclavian artery and as a result, the right subclavian artery will originate normally. B, Formation of subtype C 2IAA is shown schematically, arrow B1 shows the common site of obliteration between all of C IAA subtypes. Arrow B3 points to the right PDA that is obliterated in all of subtypes except subtype 3 and the site of obliteration of the right fourth arch, just before right dorsal aorta is shown with arrow B2, these obliterations are accompanied by opening of the dorsal aorta and aberrant right subclavian artery. C, Reveals formation of subtype 3 and arrow C1 points to the common obliteration site in all C IAA types. Arrow C3 shows right PDA, which is open in this subtype and the right subclavian artery originates from that. Arrow C2 shows obliteration of the posterior site of fourth arch just before the right dorsal aorta and therefore, causes an abnormal site for origination and formation. D, Normal obliteration sites. E, Events that happen in major arteries for formation of the abnormalities explained in this case report; arrow E1 shows the common site of obliteration in all subtypes of type C IAA. Arrow E3 shows obliteration of the right PDA and arrow E2 shows obliteration of the left dorsal aorta instead of the right dorsal aorta and as a result formation of right sided aortic arch. (The base of these schematic Figures before changes is extracted from Figure 13.37 of 12th ed. Langman’s Medical embryology 19th ed. but differences between type C IAA subtypes and normal condition and other changes are added by the corresponding author.).
Figure 4A shows the sites of obliteration that occur in subtype C1 IAA. Arrow 4A-3 points to the posterior site of the sixth arch, which is equivalent with right PDA that is obliterated normally. Obliteration of the left branch of the ascending aorta originates from the aortic sac before separation of the fourth arch, which are equivalent with type C IAA are shown by arrow 4A-1.
Arrow 4A-2 points to obliteration of the distal of the right dorsal aorta distal to the left seventh intersegmental artery, which forms on the future right subclavian artery and as a result of that, the right subclavian artery will originate normally.
In Figure 4B, formation of subtype C 2IAA is shown schematically. Arrow 4B-1 shows the common site of obliteration between all of C IAA subtypes. Arrow 4B-3 points to the right PDA that is obliterated in all subtypes except subtype 3 and the site of obliteration of the right fourth arch just before the right dorsal aorta is shown with arrow 4B-2. These obliterations are accompanied with opening of the dorsal aorta and aberrant right subclavian artery.
Figure 4C reveals formation of subtype 3 and arrow 4C-1 points to the common obliteration site in all C IAA types. Arrow 4C-3 shows right PDA which remains open in this subtype and the right subclavian artery originates from that. Arrow 4C-2 shows obliteration of the posterior site of the fourth arch just before the right dorsal aorta and thereft\ore, causes an abnormal site for origination and formation.
In Figure 4E, which shows the events happening in major arteries for formation of the abnormalities explained in this case report, arrow 4E-1 shows the common site of obliteration in all subtypes of type C IAA. Arrow 4E-3 shows obliteration of the right PDA and arrow 4E-2 shows obliteration of the left dorsal aorta instead of the right dorsal aorta and as a result formation of right sided aortic arch.
So with embryologic considerations, this case could be considered as a type C interruption and with respect to the few existing reports on IAA, particularly those similar to this case, the authors suggest that the traditional classification should be revised.