1. Background
Pulmonary embolism (PE) is a potentially fatal condition with a short-term mortality rate ranging from 1% in hemodynamically stable patients to over 90% in those with severely compromised cardiorespiratory function (1). In case of massive PE and a compromised right ventricle, the mortality rate may reach to over 50% (2). Sub-massive PE could also be complicated with right ventricular dysfunction and the prognosis may be poor (3).
Pulmonary computed tomographic angiography (PCTA) is now the mainstay imaging technique for diagnosis and risk stratification in patients with PE (4, 5). Up to now many investigators have tried to suggest PCTA-based prognostic parameters in PE, some of which originate from this assumption that the right side of the heart undergoes strain and dysfunction because of clot burden in such cases (6-8). Most of these presumably prognostic parameters, however, are controversial in terms of accuracy and reproducibility (9-12).
In one of these studies, Park et al. (13) suggested that descending aorta enhancement to main pulmonary artery enhancement (DAE/MPAE) could predict PE-related major adverse events in patients with massive pulmonary embolism. However, because of insufficient supporting studies and a potential effect of racial and regional differences on the incidence of PE-related mortality (14, 15), this conclusion needs to be verified in further investigations.
2. Objectives
The objective of the present study is to test the prognostic value of DAE/MPAE in a group of white patients with massive/ sub-massive PE.
3. Patients and Methods
3.1. Study Design and Patients
In this prospective cohort study, a total of 47 Caucasian patients with acute massive or sub-massive PE were recruited from a teaching hospital between August 2013 and December 2014. Patients without PE-related right ventricular dysfunction, with a positive history of right ventricular dysfunction, with previous PE, and with at least 3 days delay between PCTA and transthoracic echocardiography were not included. The ethics committee of our university approved this study and informed written consents were obtained from participants.
3.2. Right Ventricular Dysfunction
Any of the following findings in transthoracic echocardiography indicated right ventricular dysfunction (16): right ventricular end-diastolic diameter/left ventricle end-diastolic diameter ≥ 0.9, motion abnormality of the right ventricle wall, and tricuspid regurgitation jet ≥ 2.8 m/sec.
3.3. Massive and Sub-Massive Categorization
Patients hemodynamically stable were categorized as “with sub-massive PE” and patients with hemodynamic instability were categorized as “with massive PE”. Hemodynamic instability was present when a patient admitted with systolic hypotension (systolic blood pressure ≤ 90 mmHg) and/or syncope (13).
3.4. Outcome Measures
Requirement for endotracheal intubation and/or cardiopulmonary resuscitation during hospitalization was considered as PE-related major adverse events. Any PE-unrelated major adverse event was also documented at the same time (13). Short-term PE-related and PE-unrelated mortality was defined as expiration within 30 days post-PCTA (12).
3.5. Spiral PCTA Protocol and Variables
Contrast - enhanced, non - electrocardiography - gated spiral PCTA was performed with a 64-slice multidetector machine (SOMATOM Sensation 64, Siemens, Germany) following standard protocols (17): collimation, 1 mm; pitch, 0.8; reconstruction increment, 1 mm; rotation time, 0.33 second; 120 kV per slice; 120 - 140 mAs.
Imaging was carried out after intravenous administration of 50 mL of iopromide (Ultravist 370, Schering AG, Berlin, Germany) at a rate of 4 mL/sec, which was followed by infusion of 50 mL saline solution at the same rate. Test bolus technique was used to define scan-delay. On this basis, scanning began 5 seconds after the pulmonary artery reached 100 Hounsfield unit (HU), as described by the original study (13). CT imaging was carried out from the lowest hemidiaphragm to the top of the lungs (18).
Two attending radiologists with over 10 years of experience reviewed CT images on independent workstations. In the first place, they verified the diagnosis of PE as partial or complete occlusion of the pulmonary arteries by an endoluminal central filling defect (18). Location of the largest pulmonary artery was examined from the identified pulmonary embolism and recorded as the main pulmonary artery, lobar pulmonary artery, segmented pulmonary artery and sub-segmented pulmonary artery (13).
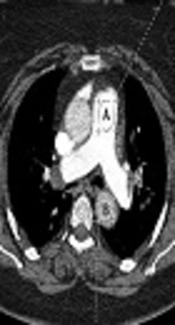
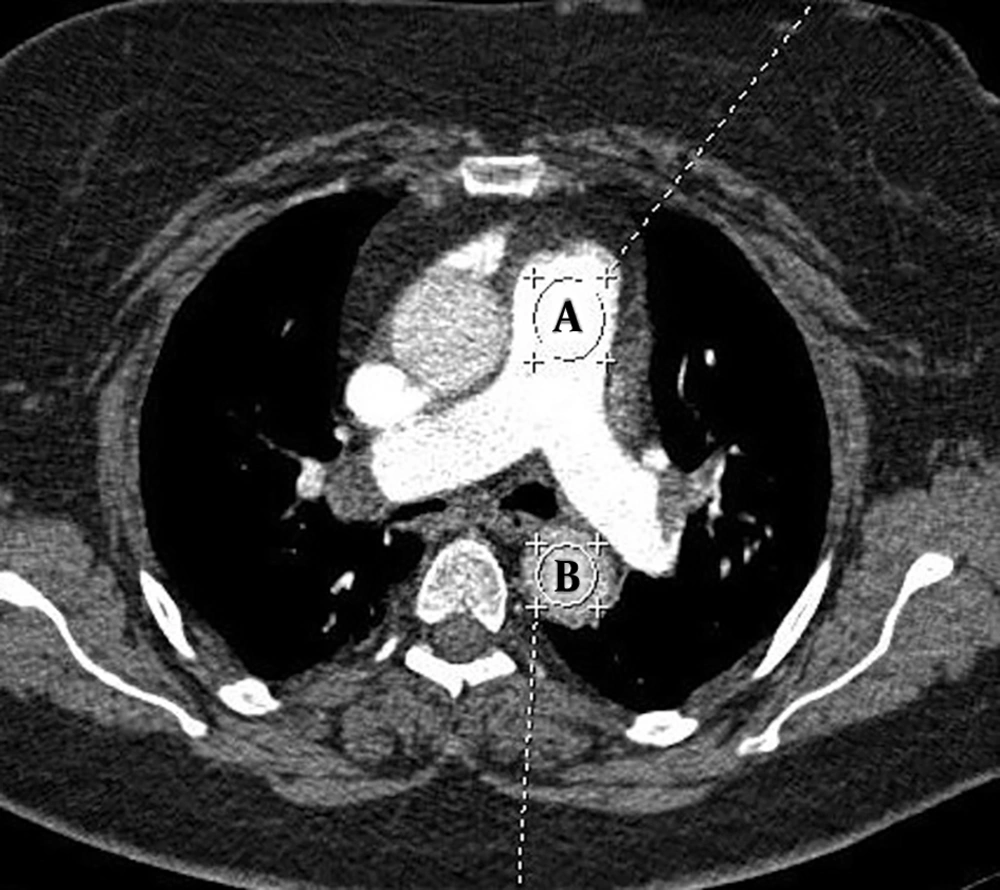
To measure the mean attenuation in vessels on trans-axial CT images, a circular region of interest (ROI) was placed on the descending aorta at the level of pulmonary bifurcation and on the main pulmonary artery (Figure 1) (13). The pulmonary artery obstruction score (PAOS) was determined according to the method suggested by Qanadli et al. (19) for assessment of clot burden.
3.6. Statistical Analysis
SPSS software version 19.0 (IBM Corp. Released 2010. IBM SPSS Statistics for Windows, Version 19.0. Armonk, NY) was used for statistical analysis. Independent samples t test, Mann-Whitney U test and Kruskal Wallis test were used where appropriate. Pearson coefficient (r) was calculated to examine correlations between study variables. The intraclass correlation coefficient was used to assess inter-observer variability. The significance level was set at P ≤ 0.05.
4. Results
Patients’ demographic data are summarized in Table 1. The mean reported DAE by the first observer was 135.34 ± 61.14 (range, 62.40 - 376.00) and the mean reported DAE by the second observer was 136.01 ± 96.31 (range, 61.00 - 379.00). The intra-class correlation coefficient was very high (0.99) for the two readings. The mean DAE by the two observers was 135.68 ± 61.12. The mean reported MPAE by the first observer was 397.29 ± 96.31 (range, 156.00 - 690.00) and the mean reported MPAE by the second observer was 397.16 ± 95.69 (range, 156.00 - 686.00). Again, a very high intra-class correlation coefficient (0.99) was present between the two readings. The mean MPAE by the two observers was 397.16 ± 95.69. The mean DAE/MPAE was calculated at 0.36 ± 0.19 (range, 0.13 - 0.99).
| Variable | Datum |
|---|---|
| Sex | |
| Male | 21 (44.7) |
| Female | 26 (55.3) |
| Age, y | 61.72 ± 14.53 (29 - 83) |
| Presenting complaint | |
| Dyspnea | 16 (34) |
| Dyspnea and hypotension | 16 (34) |
| Dyspnea and chest pain/discomfort | 4 (4.5) |
| Hypotension | 4 (8.5) |
| Dyspnea and mental change | 2 (4.3) |
| Dyspnea and hypotension | 2 (4.3) |
| Chest pain/discomfort | 1 (2.1) |
| Syncope | 1 (2.1) |
| Hypotension and chest pain/discomfort | 1 (2.1) |
| PE extent | |
| Massive | 24 (51.1) |
| Sub-massive | 23 (48.9) |
| PE location | |
| Main | 31 (66) |
| Lobar | 13 (27.7) |
| Segmental | 3 (6.4) |
| PAOS (Based on Qanadli method), % | 11.81 ± 2.15 (5 - 19) |
| Thrombolysis | 45 (95.7) |
| Embolectomy | 5 (10.6) |
| Hospital stay, day | 11.63 ± 6.11 (1 - 30) |
Abbreviations: PAOS, Pulmonary Artery Obstruction Score; PE, Pulmonary Embolism; y, year.
aData are presented as mean ± SD (range) or frequency (%).
In hospital endotracheal intubation and cardiopulmonary resuscitation (CPR) were performed in 13 (27.7%) and five (10.6%) patients, respectively. The 30-day PE-related mortality rate was 25.5% (n = 12), with no PE-unrelated death occurred during the same period of time. PE-unrelated major adverse events occurred in five patients (10.6%), including sepsis (n = 2), intraventricular hemorrhage (n = 1), gastrointestinal hemorrhage (n = 1) and acute renal failure (n = 1).
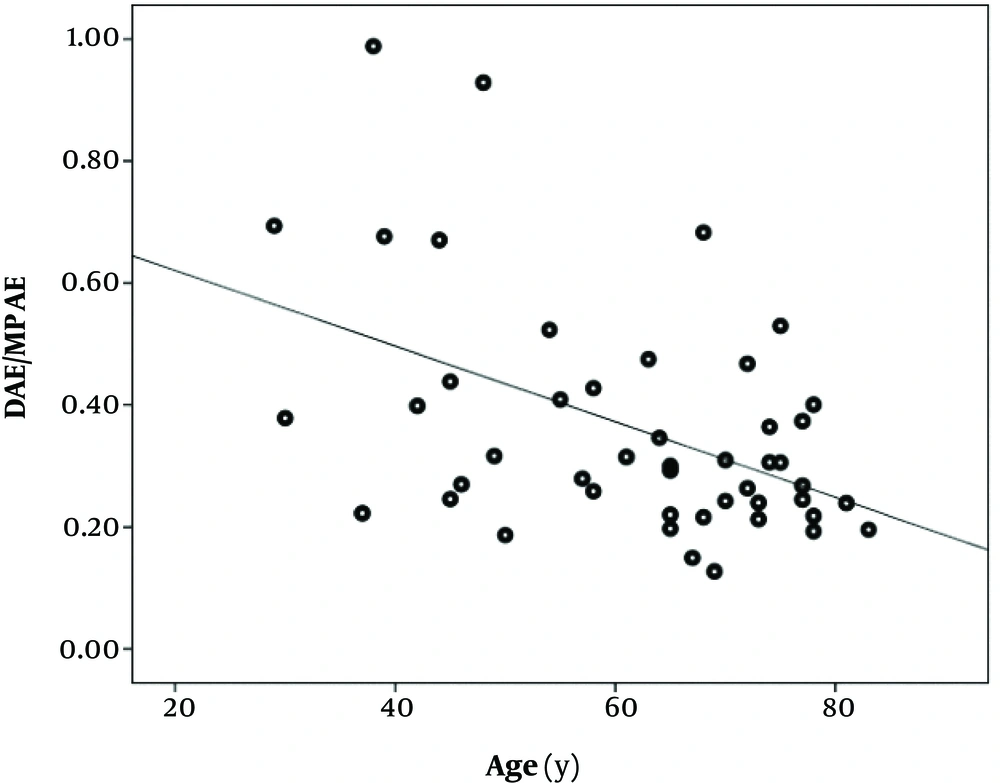
A significant, reverse correlation was present between DAE/MPAE and patients’ age (Pearson r = -0.47, P < 0.001) (Figure 2). No significant correlation was observed between DAE/MPAE and PAOS (Pearson r = -0.03, P = 0.86), and between DAE/MPAE and hospital stay (Pearson r = 0.16, P = 0.33). DAE/MPAE values stratified by study variables are set out in Table 2. No significant association was found in this regard.
| Variable | No. of patients | DAE/MPAE | P value |
|---|---|---|---|
| Sex | 0.27* | ||
| Male | 21 | 0.33 ± 0.19 | |
| Female | 26 | 0.39 ± 0.19 | |
| PE extent | 0.77* | ||
| Massive | 24 | 0.35 ± 0.19 | |
| Submassive | 23 | 0.37 ± 0.19 | |
| PE location | 0.41* | ||
| Main pulmonary artery | 31 | 0.35 ± 0.16 | |
| Other | 16 | 0.39 ± 0.24 | |
| Endotracheal intubation | 0.31 | ||
| Negative | 34 | 0.31 (0.23) | |
| Positive | 13 | 0.26 (0.17) | |
| Cardiopulmonary resuscitation | 0.89 | ||
| Negative | 42 | 0.30 (0.21) | |
| Positive | 5 | 0.38 (0.21) | |
| Mortality (30-day) | 0.63 | ||
| No | 42 | 0.31 (0.18) | |
| Yes | 5 | 0.24 (0.34) | |
| PE-unrelated major adverse events | 0.47 | ||
| Negative | 42 | 0.31 (0.20) | |
| Positive | 5 | 0.25 (0.30) | |
| PE-related major adverse events/mortality | 0.47 | ||
| Negative | 32 | 0.31 (0.22) | |
| Positive | 15 | 0.26 (0.26) |
aData are presented as mean ± SD or median (interquartile range).
bIndependent samples t test (*) or Mann-Whitney U test are used for comparisons.
cP value < 0.05 is statistically significant.
The median PAOS was significantly higher in patients with PE-related in-hospital major adverse event/30-day mortality compared to patients without PE-related in-hospital major adverse event/30-day mortality (5.00 with interquartile range [IQR], 6.00 vs. 12.00 with IQR, 8.00; P = 0.03).
The rate of massive PE was significantly higher in patients who underwent intubation compared to those who did not (92.3% vs. 35.3%; P < 0.001), in patients who underwent CPR compared to patients who did not (100% vs. 45.2%; P = 0.05), in patients who expired within 30 days after PCTA compared to patients who did not (100% vs. 45.2%; P = 0.05), and in patients with bad outcome in general compared to those without (92.3% vs. 35.3%; P < 0.001).
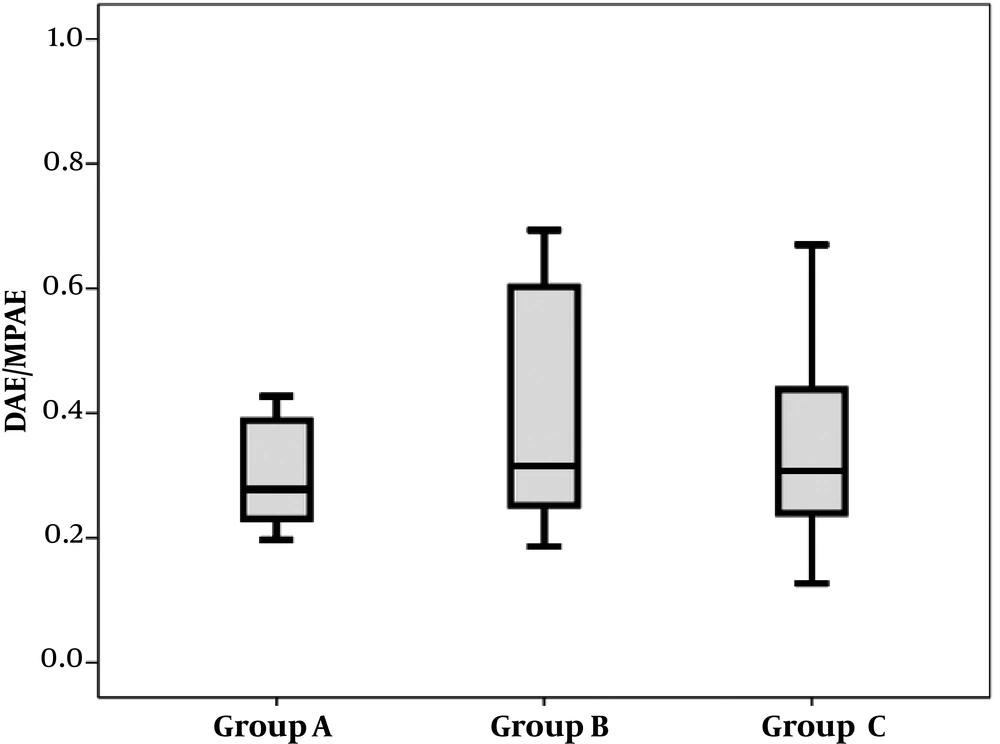
The median DAE/MPAE was 0.28 (IQR, 0.17) in patients with massive PE and unfavorable outcome (n = 12), 0.32 (0.39) in patients with massive PE and favorable outcome (n = 12) and 0.31 (0.21) in patients with sub-massive PE and favorable outcome (n = 22). The three groups were comparable in this regard (P = 0.63) (Figure 3).
Box plots of descending aorta enhancement to main pulmonary artery enhancement (DAE/MPAE) ratio in patients with massive pulmonary edema (PE) and unfavorable outcome (group A), in patients with massive PE and favorable outcome (group B) and in patients with sub-massive PE and favorable outcome (group C).
5. Discussion
In the present work, the two groups of patients with and without PE-related adverse events/ mortality were comparable in terms of the median DAE/MPAE. For the first time in 2012, Park et al. (13) suggested that DAE/MPAE might predict PE-related major adverse events in patients with massive pulmonary embolism. The authors assumed that in case of severe PE and right ventricular dysfunction more contrast agents are retained in the pulmonary circulation, leading to decreased DAE and increased MPAE.
Most likely this assumption dates back to 1998, when Miller et al. (20) suggested that the reflux of contrast dye into the inferior vena cava might be a sign of right-sided heart dysfunction. Later on, Kang et al. (21) confirmed their findings, but Collomb et al. (22) found no prognostic role for the reflux of contrast dye into the inferior vena cava in PE patients. In line with our finding, a recent study by Hefeda and Elmasry (23) on 32 patients with PE also documented no significant difference between survivors (n = 23) and non-survivors (n = 9) in terms of the mean DAE/MPAE.
Many factors may affect contrast enhancement including the amount of injected contrast material, flow rate, and in particular, the scan delay (24-26). It should be noted that empiric bolus timing could be difficult especially when the pressure of pulmonary artery is elevated and poor performance of the right cardiac side is present (26). In addition to these parameters, the presence of bronchopulmonary collateral vessels in some patients with previous chronic inflammatory diseases may serve as an extensive left-to-right shunt, and affects the attenuation of the pulmonary vessels (25, 27). A preexisting patent foramen ovale may also cause insufficient attenuation of the pulmonary arteries. Although this condition may seem rare, in a previous study patent foramen ovale caused abnormal contrast dynamics in 16% of patients with suspected PE (26). Other miscellaneous factors that may cause inadequate pulmonary artery enhancement in PE patients are air space consolidations, intracardiac shunts, preexisting undiagnosed heart failure, high cardiac output, and obstruction of the superior vena cava (24-26, 28).
Besides contrast material administration, respiration may also dramatically influence scan quality in PE patients, because the dedicated guideline of breathing during computed tomographic scanning is usually hard to follow by patients with suspected massive or submassive PE (24, 25, 29).
It has been suggested that DAE/MPAE could be obtained more objectively and more reliably than similar previously defined prognostic factors in association with right ventricular dysfunction (13). We also showed very low inter-observer variation in reporting DAE/MPAE in the present work. On the basis of discussed parameters in association with attenuation of the pulmonary vasculature, however, a considerable variation could be expected with employment of DAE/MPAE as a prognostic factor, as the present study confirmed.
It has been proposed that a mechanical obstruction by the intravascular clot is not the sole contributor to pulmonary vascular resistance and other factors such as systemic arterial hypoxemia, reflex vasoconstriction and release of vasoactive agents may also come to play (30). In addition, many clots lodge in small peripheral pulmonary arteries with no significant contribution to overall obstruction in pulmonary vasculature in PE (31). Therefore, the degree of enhancement of pulmonary vasculature in PE may not reflect actual severity of the obstruction. An insignificant correlation between DAE/MPAE and PAOS in the present work supports this surmise.
Finally, we found a significant reverse correlation between patients’ age and DAE/MPAE. Age is a known risk factor for untoward consequences in patients with PE (32). In a recent study, our group found age as the only prognostic factor in patients with PE, which was independent of right heart failure and PAOS (33). In the international cooperative pulmonary embolism registry, age of > 70 years has been suggested as a negative prognostic factor in patients with PE (34). At the same time, it has been shown that an increasing age aggravates right ventricle (RV) dysfunction (35). Accordingly, it could be suggested that a significant association between DAE/MPAE and patients’ outcome in PE is only the effect of age. Unfortunately Park et al. did not examine age as a prognostic factor in their study. The mean age of their patients (71.4 years), however, was significantly more than that in the present work (61.7 years) and in Hefeda’s series (56.8 years).
Our sample size is larger than that of the original report (13), but for more definite prognostic purposes, larger sample sizes should be studied in future works. In addition, although a 3-month follow-up encompasses the critical period in patients with PE (3), longer follow-ups are needed to fully examine the prognostic importance of DAE/MPAE.
As mentioned earlier, some pathologies such as air space consolidations, intracardiac shunts, preexisting undiagnosed heart failure, high cardiac output, and obstruction of the superior vena cava may affect the prognostic value of DAE/MPAE in PE. Although many of these conditions are rare, more controlled studies are needed to reach a definite conclusion in this regard.
Finally, accurate separation of PE-related and unrelated complications/death is impossible and this may limit drawing a solid conclusion in examining the prognostic value of DAE/MPAE in PE. It should be noted, however, that in the present study we showed significant associations between the extent (and severity) of PE and PE-related complications/mortality, which may, at least partly, confirm the accuracy of proposing those outcome variables as PE-related ones.
In summary, this study showed that DAE/MPAE on PCTA could not be considered as a reliable short-term prognostic factor in patients with massive or sub-massive PE.