1. Introduction
Juvenile fibroadenoma, which is an uncommon variant of fibroadenoma, occurs predominantly in adolescents. It is characterized by increased stromal cellularity and constitutes approximately 7-8% of all fibroadenomas. The development of ductal carcinoma in situ (DCIS) within a fibroadenoma is a rare clinical condition (1, 2). There have been several reports of carcinomas arising within fibroadenomas (3-6); however, features of mammography, sonography, and dynamic contrast-enhanced breast MRI (DCE-bMRI) have not been well described. In the present report, we describe a case of DCIS in juvenile fibroadenoma focused on preoperative imaging findings of mammography, sonography, and DCE-bMRI with diffusion weighted imaging (DWI).
2. Case Presentation
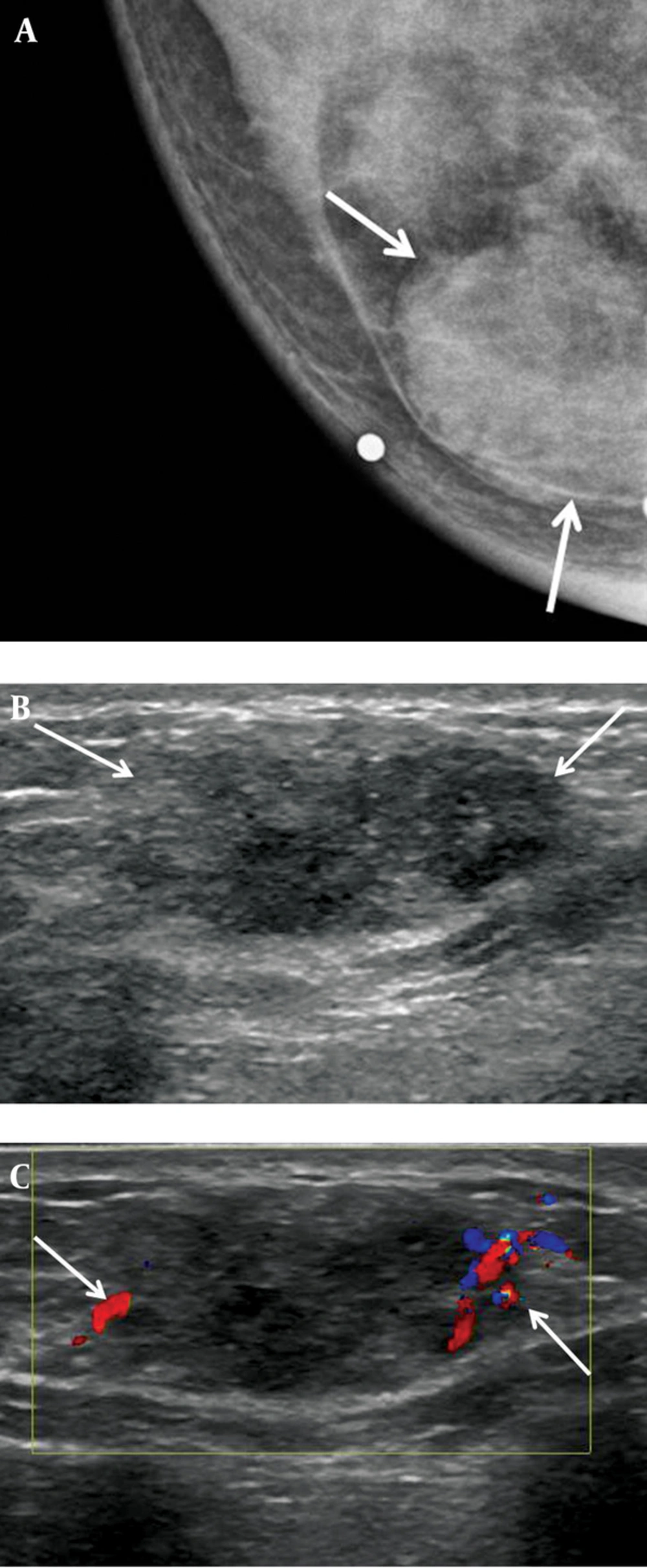
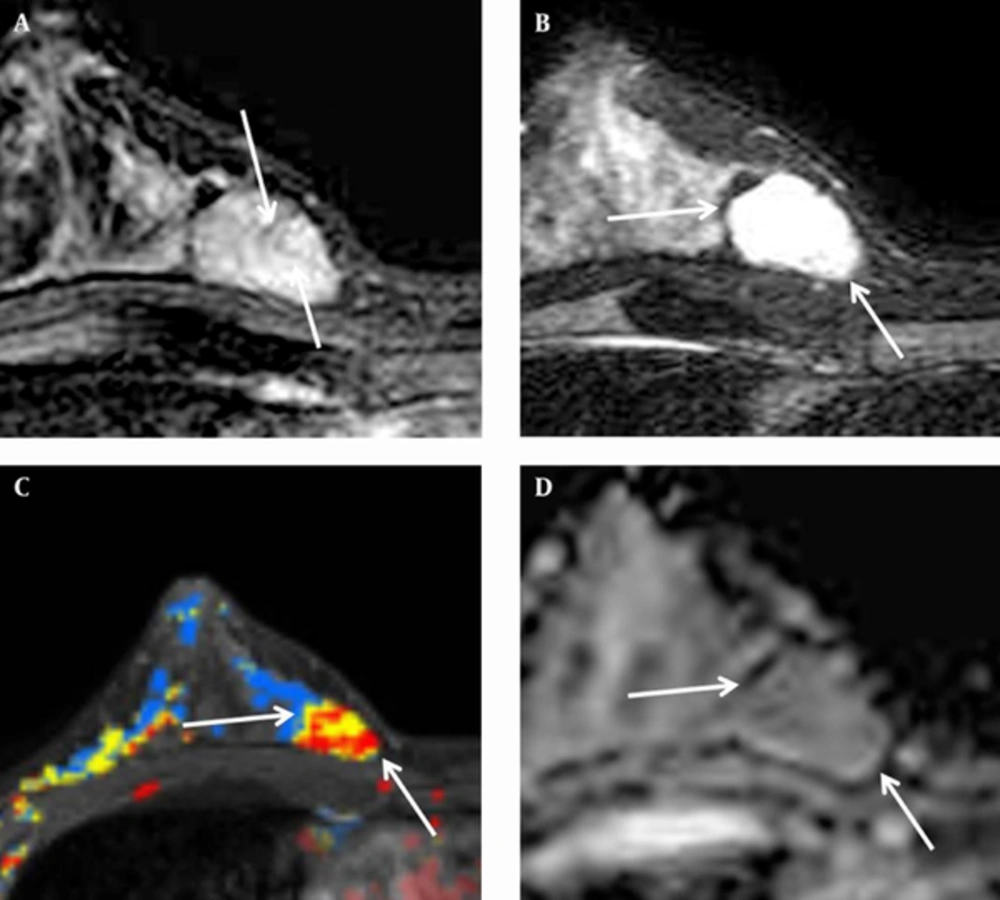
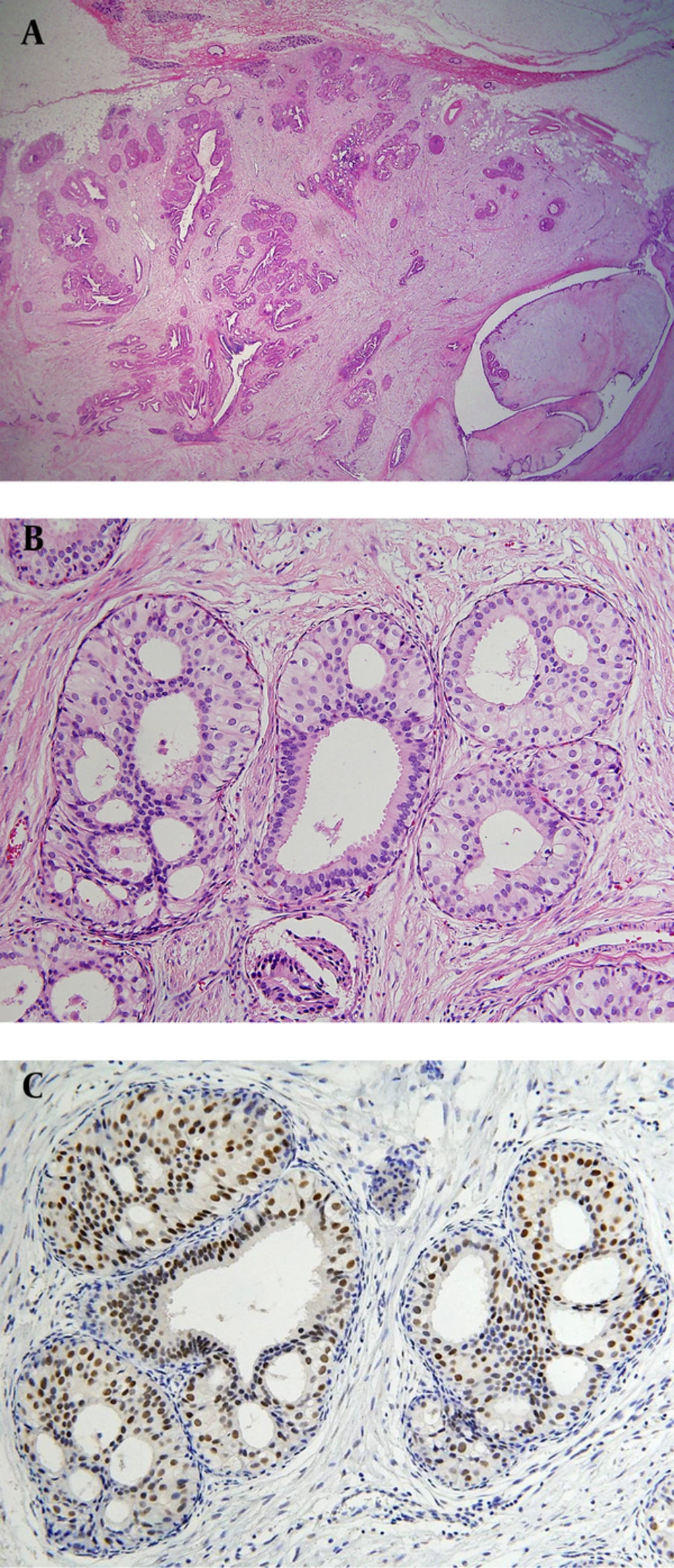
A 36-year-old woman, gravida 2, para 2, had a palpable mass in the mid inner portion of the right breast. The mass had been present for several months and was painful. The patient had a history of focal excision for fibroadenoma in the upper inner quadrant of the right breast in another hospital. The patient had no family history of breast cancer. Skin retraction, nipple discharge or palpable lymph nodes were not observed on clinical examination. On mammography (Figure 1 A), a 2-3 cm in diameter, circumscribed, oval-shaped, isodense mass was seen in the right inner breast on the craniocaudal view. This mass was not detected on the mediolateral oblique view. Microcalcifications were not detected on mammography. Ultrasonography (Figure 1 B) showed an indistinct, 2.3 × 1.1 × 1.7 cm, oval shaped, heterogeneous and mild hypoechoic mass in the palpable area. On power Doppler study, a blood vessel was seen in the periphery of the mass (Figure 1 C). Breast MRI was performed using a 3.0 T -MR imaging system (Achieva 3.0 T TX; Philips Healthcare) with a dedicated phased-array breast coil, and the patient in the prone position. DWI was acquired in the transverse plane and covered both breasts. A spin-echo single-shot echo-planar-imaging sequence with diffusion-sensitizing gradients was applied along orthogonal direction. These images were used to synthesize isotopic transverse images (repetition time ms/echo time ms, 5417/72; b values, 0, 600 and 1000 s/mm2; image matrix, 96 × 126; field of view, 320 × 320 mm; section thickness, 3 mm; section gap, 0 mm; three signal acquired; acquisition time, 80 s). After DWI, T2-weighted fast spin-echo transverse images were also obtained. The following image parameters were used: 5727/70; flip angle, 90°, image matrix, 620 × 309; field of view, 581 × 342 mm; section thickness, 3 mm; section gap, 0 mm. A three-dimensional T1-weighted fast spoiled gradient-echo sequence was also performed with transverse imaging using one pre-contrast and six post-contrast dynamic series, immediately after contrast injection and after 60, 120, 180, 240, and 300 seconds. The image parameters were as follows: 6/3; flip angle, 0°; image matrix, 436 × 436; field of view, 330 × 340 mm; section thickness, 3 mm; section gap, 1.5 mm. Gadoterate meglumine (Dotarem; Guerbet, Villepinte, France) was injected per kilogram of body weight into an antecubital vein with a power injector (Spectris; Medrad, Indianola, PA, USA) at a rate of 2 mL/s. DCE-bMRI revealed a smooth-marginated, heterogeneously well-enhanced mass (Figure 2 A), which demonstrated high signal intensity on T2-weighted imaging (Figure 2 B) and low signal intensity on T1-weighted imaging. This showed early rapid enhancement and delayed washout enhancement (Figure 2 C) on the kinetic analysis in the majority of the mass. This mass shows heterogeneous high signal intensity on DWI with high b-value (b = 1000) without definite diffusion restriction (Figure 2 D). The patient underwent breast-conserving surgery for the palpable mass. Histopathological examinations (Figure 3) showed multiple foci of atypical ductal hyperplasia and ductal carcinoma in situ, with a cribriform or solid pattern, within a juvenile fibroadenoma. In the DCIS areas, most ductal epithelial cells showed overexpression of estrogen receptor (ER) immunostaining, and p63-positive myoepithelial cells were preserved only in the periphery. There was no evidence of metastasis in sentinel lymph nodes. Radiation therapy was performed in the right breast after surgery. The patient had no evidence of recurrence 12 months after the surgery.
A 36-year-old woman with low-grade ductal carcinoma in situ (DCIS) arising within a juvenile fibroadenoma. A, Craniocaudal view mammography of the right breast reveals a relatively circumscribed mass (arrows) without internal microcalcification. B, Ultrasonography of the right breast shows an oval-shaped heterogeneous mild hypoechoic mass (arrows) with an indistinct margin. C, Color Doppler sonography shows increased vessel flow (arrows) in the periphery of the mass.
A, Axial dynamic early subtraction image of dynamic contrast-enhanced breast MRI shows an early peak enhancing mass with internal non-enhancing septa (arrows) in the right mid inner breast. B, Fat-suppressed axial T2-weighted imaging shows high signal intensity of the mass (arrows). C, Computer-aided detection (CAD) assessment of the mass at the 50% threshold level reveals early rapid and delayed wash out enhancement (red color) in the majority of the mass (arrows). D, Apparent diffusion coefficient (ADC) map of diffusion weighed images reveals that there is no definite diffusion restriction.
Histopathologic examinations after surgery. A, On low power field microscopy (12 ×), the lesion is a well-demarcated ovoid mass with a fibroadenomatoid area in the right lower portion, which shows a pericanalicular growth pattern with diffuse increase in stromal cellularity. B, Multiple foci of ductal carcinoma in situ (DCIS) with a cribriform pattern is seen within the mass. C, On estrogen receptor (ER) immunostaining, most of the ductal epithelial cells show overexpression in DCIS areas.
3. Discussion
Fibroadenoma is a benign fibroepithelial tumor and not generally considered as a risk factor for breast cancer. Dupont et al. reported that the risk of breast cancer is increased in women with complex fibroadenomas, proliferative disease, or a family history of breast cancer (7). The occurrence of a carcinoma evolving within a fibroadenoma is rare, with an incidence of 0.02% (8). The mean age of patients with a carcinoma arising in a fibroadenoma is within the fifth decade, which is up to 20 years older than patients with simple fibroadenoma (1). Diaz et al. reported that the predominant type of malignancy in fibroadenoma was carcinoma in situ (95%), with equal distribution between lobular carcinoma in situ (LCIS) and DCIS (1). A few studies have been reported for imaging findings of carcinoma arising from a fibroadenoma. Mammographic features such as indistinct margins and clustered microcalcifications within the mass raise the suspicion of malignancy (3, 9). Ultrasonographically, a focal increase of color flow signals within the mass on color Doppler study gave rise to a suspicion of malignancy (6). In our case, microcalcification was not detected on mammography. Increased vascularity was seen in the periphery of the tumor mass on color Doppler sonography. However, carcinoma in situ was present in the majority of the mass. Typically, on MRI, fibroadenoma appears as a round or oval-shaped mass with a smooth margin. Fibroadenoma can exhibit variable enhancement; however, enhancement usually persists until the delayed phase (4). Only few reports described DCE-bMRI findings of carcinoma within fibroadenoma. Shin et al. reported a mass showing a pattern of early peak enhancement in the central area and delayed rim enhancement in the periphery, and in histology, DCIS was found in the center of the fibroadenoma (5). Kato et al. described that the area of DCIS within the fibroadenoma showed rapid initial enhancement and washout kinetics on DCE-bMRI; whereas, the major part of the mass was consistent with fibroadenoma (4). These findings are concordant with our case, which showed early rapid and delayed wash-out enhancement in the majority of the mass. A preoperative diagnosis of malignancy in fibroadenoma can be difficult. In the present case, an indistinct margin of the mass on sonography and a type III enhancement pattern on DCE-bMRI were the clues of malignancy. Histologically, the mass showed DCIS almost entirely within the fibroadenoma. Increased color flow signals were noted only in the periphery of the mass on color Doppler sonography. In conclusion, preoperative detection of malignancy developing within a fibroadenoma can be difficult. In a patient with a newly developed mass and palpability, further management, including core needle biopsy, should be considered, especially for any suspicious findings that are present such as increased color flow signals on color Doppler sonography or type III kinetic curve on DCE-bMRI.