1. Background
The first line of treatment for patients suffering from mild and non-critical intermittent claudication is modification of possible risk factors and refining lifestyle and medication (1). However, more severe aortoiliac lesions causing critical symptoms are in need of more invasive treatments.
Lately, there has been the trend to use endovascular treatment (ET) widely since it has been shown to have promising outcomes in different studies although further studies are suggested (2-8). Due to the limited number of studies and absence of unanimous decision on the best modality to be used and patient criteria, new techniques for ET in occlusive aortoiliac lesions are introduced and many show desirable results for special patient settings (3, 9).
The trans-atlantic inter-society consensus for the management of peripheral arterial disease (TASC II) document classifies bilateral infra-renal aortoiliac and unilateral iliac occlusions from origin to after internal iliac artery as type D lesions. For the mentioned lesions, the current recommendation is to use open surgical procedure (bypass graft) (1) with a patency rate of 87.5% and 81.8% at 5 and 10 years in ischemic limb patients but more importantly mortality rate and morbidity rate were 3.3% and 8.3% in the studies (10). However, use of ET for such lesions is not uncommon as many patients who need the intervention do not meet the essential criteria for surgery (8).
2. Objectives
The purpose of this study was to evaluate the results of a newly introduced procedure and to discuss the possibility of using ET instead of open surgery in the selected cases. Speaking of total bilateral aortoiliac occlusion (a subclass of TASC II type D lesions), the literature seems to lack sufficient number of studies focusing specifically on this subclass of TASC II type D lesions. Therefore, in the current study we tried to bring attention to total bilateral aortoiliac lesions.
3. Patients and Methods
The current study was approved by the ethics committee of Tehran University of Medical Sciences. During the period of May 2014 until May 2016, patients who came to the vascular clinic of Sina hospital with chronic lower limb ischemia signs in terms of Rutherford classification (10) of grade I or higher and absence of femoral pulses bilaterally, were screened with multi-slice CT angiography and all of those with total infra-renal bilateral aortoiliac occlusion (a sub category TASC II type D) were brought into the study. Patients were categorized according to Rutherford classification (10). Patients with intermittent claudication were first treated with exercise therapy. Those individuals who were suffering from life disturbing claudication even after initial treatment, a chronic progression to rest pain following claudication, and limb ulcers were candidates for the treatment. Patient data was prospectively gathered. Those patients with acute limb ischemia signs and those with a known history of sensitivity to contrast agent or elevated serum creatinine levels were excluded. Informed consent was acquired from patients who were enrolled in the study.
All patients were assessed by anesthesia and cardiology services as part of preoperative preparation. Patients were prepared and treated at Sina hospital and Sina hospital catheter lab. The procedure details and possible side effects were fully explained to the patients and informed consent was acquired. On the intervention day, routine laboratory tests and reservation of iso group and iso RH packed cell units was done and each patient underwent complete preparation of the anterior thorax, abdomen, both groins, and left arm. In the catheter lab, an expert anesthesiologist completely monitored the patient during the procedure and for 2 hours post intervention.
Access to the left brachial artery was obtained using a 5 French (F) sheath. Aortic and lower limb angiography was performed after 0.035 hydrophilic regular guide wire introduction into the aorta with a Judkins Right (JR) catheter and then by replacing it with a pigtail catheter with assistance of an injector. Angiography was used to locate renal arteries, aortic cutoff, each side lower limb run off, and evaluation of distal arteries of the limbs. Then, 5F sheath was replaced with 7F sheath (65 cm or 90 cm in length according to patient height) after parking standard wire in the aorta. At this point, 5000 international units (IU) intravenous (IV) heparin was injected and 450 mg clopidogrel was administered. Afterward, using MP catheter, passage of 0.035 hydrophilic regular guide wire to the one side of aortoiliac occlusion at one limb was tried. A balloon, 8 mm in diameter and 40 cm in length, was used at the aorta and a stent with 14 mm diameter and 40 length (nitinol bare stent) was opened there. Then, a 12 mm diameter and 40 mm length balloon was inflated intra-stent at aorta. Using a smaller balloon after stent placement and gradual expansion of Nitinol stents to their true size can lower the risk of rupture. Next, at the other side limb, a 7F short sheath was inserted at the common femoral artery (CFA) under the guidance of sonography or fluoroscopy. Note that in this procedure only one femoral access is needed. MP catheter and 0.035 hydrophilic regular guide wire is used to cross the occlusion to the descending aorta from CFA. Number 5 balloon was then simultaneously inflated at both iliac arteries and 7 mm stents (100 mm or 150 mm in length depending on iliac occlusion length) were opened at each iliac artery, continued with inflating another number 6 balloon in the iliac stents. Finally, aortogram and lower limb angiography for confirmation were done to check if sufficient distal flow and resolution of stricture were achieved (Figure 1). One or two hours later, the sheaths were taken out. Manual compression was used for homeostasis. The patient was transferred to the vascular surgery ward to be under observation for the following 48 hours. In case of no problematic event, the patient was discharged. The average time for treatment was 120 minutes. For follow-up, during the first month after the procedure, weekly evaluation using physical examination and sonography at the vascular surgery clinic was carried out. If strong femoral pulses, three-phasic flow in femoral artery, and recovery from critical limb ischemia symptoms in terms of no more rest pain were present, quarterly visits were recommended thereafter. During each visit, the treatment process consisting of femoral pulse examination, improvement of disease signs and symptom evaluation and patient satisfaction number (in terms of patient’s overall satisfaction from the procedure on a visual 10 scale with 10 being the best) was performed and documented. In case any suspicion of suboptimal stent performance such as poor femoral pulses, relapse of symptoms, or unfavorable blood flow was present, CT-angiography was requested and if re-stenosis was established, re-angiography through brachial artery was performed and the strictures in the stents underwent angioplasty with drug ballooning. Brachial access was preferred since it could result in better angiography and therapeutic procedure when both common and external iliac arteries were stented previously.
Patient data at 3, 6, and 12 months after the intervention were recorded in specially prepared forms and demographic characteristics, satisfactions and patency rates were calculated and 95% confidence intervals (CI) using binomial exact test were made and activity level before and after treatment were compared using paired sample t-test.
4. Results
Among screened patients, 15 met the inclusion criteria and entered the study. All patients underwent endovascular therapy as explained before. Patients mean age was 59.9 (range: 45 to 77) years, consisted of 12 men and 3 women (Female: 20%, Male: 80%). Among all, 66.7% (10 of 15) were smokers and 66.7% (7 of 10) of the smokers stopped smoking after the treatment. Concomitant coronary artery disease was present in 73.3% (11 of 15) and diabetes mellitus was positive in 53.3% (8 of 15) of the population. Demographic information is presented in Table 1.
| Variable | |
|---|---|
| Age (mean ± SD) | 59.9 ± 10.6 |
| Gender, N | |
| Male | 12 |
| Female | 3 |
| Smoking,N | |
| Prior To ET | 10 (66.7%) |
| Continued After ET | 3 (20%) |
| CAD, N | 11 (73.3%) |
| DM, N | 8 (53.3%) |
| Activity Level Before ET, N | |
| Extremely Inactive (1) | 7 |
| Sedentary (2) | 8 |
| Moderately Active (3) | 0 |
| Vigorously Active (4) | 0 |
Patients' Demographic Data
In all 15 cases, local anesthesia was used. Technical success, in terms of no residual obstruction or no more than 30% stricture at the lesion site and sufficient distal blood flow observed in post stenting final angiography, was achieved in every patient (n = 15 [100%]). Ankle brachial index (ABI) was not used as an index since it might increase slowly after the procedure, in addition to inter- and intra-observer errors and low sensitivity to detect post-procedure ischemia and thrombosis (11). All procedures were followed according to our protocol without problem. No stent compression or fractures were seen and distal embolization was totally absent. None of the patients needed any concomitant bypass surgery. Manual compression was used for homeostasis. Distal arterial disease defined as atherosclerotic abnormalities at superficial femoral artery, popliteal artery, anterior tibial artery, and posterior tibial artery was observed in 10 (66.7%) patient’s angiographies. Mean and median hospital stay were 5.1 and 3 days (range: 2 to 14 days).
No patient experienced hematoma at either upper or lower limb access points or, brachial or femoral occlusions. Two patients (13%) had early mortality (during the first week) after therapy. The first mortality happened in a 54-year-old male patient 2 days after surgery. A long time before ET, the patient suffered from congestive heart failure. ET was performed because severe gangrene and tissue loss was present. The other patient was a 66-year-old female with severe ischemic heart disease and previous cerebral vascular accidents who had a bad medical status. She experienced respiratory distress and pleural effusion 2 days after the procedure. She passed away at day 4 because of MI although long CPR was performed. The patient awarely chose the procedure because it was limb-saving. The last event of death during our follow-up time was at 13 months post treatment which happened in a 56-year-old man again due to MI, but his stents were patent until then. Death was apparently irrelevant to our procedure because no related complaint or symptoms of thrombosis or re-occlusion were present. Acute thrombosis was seen in 1 patient defined as emergence of no improvement of signs and symptoms until 7 days after treatment. Therefore, antibiotics were administered. Reluctantly, amputation because of severe infection was performed at day 10. We believe that acute thrombosis was a result of severe distal arterial disease and poor run-off.
The mean follow-up time was 11.7 (range, 3 - 25) months. Activity levels before and 3 months after surgery were recorded among patients with patent stents according to Food and Agriculture Organization of United Nations classification (12) as 1.53 and 2.58 (P < 0.001). Satisfaction was measured with a mean of 7.9 out of 10 (SE: 0.5). Primary patency rates, in means of percentage of patients with more than 50% patent diameter, at 3, 6, and 12 months were 92.3% (24 of 26 limbs, CI = [74.7, 99.0]), 90.9% (20 of 22 limbs, CI = [70.8, 98.9]), and 83.3% (15 of 18 limbs, CI = [58.6, 96.4]), respectively. For 12 months follow-up, secondary patency rate increased to 88.9% (16 0f 18 limbs, CI = [65.3, 96.4]). Secondary patency includes patients with either primary patency or those who regained patency after the second procedure. A case of unilateral occlusion additionally happened at 11 months after treatment that was reopened using intra-stent drug ballooning. The results are summarized in Table 2.
| Variable | 3 Months | 6 Months | 12 Months |
|---|---|---|---|
| Activity level after procedure | |||
| Extremely inactive (1), N | 2 | ||
| Sedentary (2), N | 2 | ||
| Moderately active (3), N | 8 | ||
| Vigorously active (4), N | 1 | ||
| Mean | 2.58 (vs 1.53 P < 0.001) | ||
| Complication | |||
| MI, N | 2 | ||
| Acute stent thrombosis, N | 1 | ||
| In stent restenosis, N | 1 out of 18 limb | ||
| Patency rate, % | 92.3 | 91 | Primary = 83.3 and secondary = 88.9 |
Results (Activity Level, Complications, and Patency Rate)
5. Discussion
Endovascular therapy is a new evolving method. Due to its less invasive nature, fewer complications, and shorter hospital stay, ET is getting more and more popular among patients and health service providers (4). On the other hand, according to the trans-atlantic intersociety consensus (TASC) II recommendations concerning type C and D lesions, the first choice of treatment should be surgery (aortofemoral bypass) as the gold standard (1). The aforementioned procedure might not always be feasible, in as much as, patients are mostly aged and there are several contraindications for surgery. Therefore, a smaller number of people can benefit from surgery compared to endovascular therapy. Moreover, there are different studies that confirm safety, efficacy, and promising outcomes of endovascular therapy, although the number of studies is limited and insufficient (2, 8). Different methods such as using bare stents, covered stents or different placement methods have been studied but more thorough evaluations are yet suggested (3, 8, 13-17). As mentioned before, the rate of utilizing this method among different centers has been ascending during the past few years (18).
The first thing that matters in view of some experts is the possibility of using endovascular method instead of surgery to meet the need of more patients and to decrease mortality and morbidity of the treatment, yet achieving acceptable results. So, comparing these two methods is crucial to our study. In our study, we found a 83.3% primary patency rate in one year. Lun et al. reported a 93.6% patency rate for the similar time period (19). However, our result does not have a significant difference from theirs (83.3% vs. 93.6%; P value = 0.409). In another study conducted by Psacharopulo et al., a comparison was made between two groups who underwent either endovascular repair (EVR) or open surgery repair (OSR) for TASC II type D lesions (8). Two-year patency rate for EVR and OSR was 94% and 97%, respectively, meaning that no significant (P = 0.50) difference was present between the groups. However, what drew attention was the frequency and diversity of complications in the two groups. In EVR group, in the 33 limbs that were treated, only two cases faced unilateral occlusion after the procedure, both of which were managed and treated well with no serious problems afterwards (at 19 months). In the OSR group, two self-limited acute renal failures were seen out of 36 limbs, one unilateral occlusion at 19 months, and one bilateral occlusion at 33 months, and one case of pneumonia at the second day after surgery was seen. Two patients who were on double anti-platelet therapy underwent surgery again due to hemoperitoneum and one incisional hernia happened at 18 months that needed surgery. Just like the EVR group, in our study, there were no major complications but one acute unilateral thrombosis which we believe happened due to the presence of distal disease and insufficient outflow. The deceased cases all had severe concomitant diseases and surgery was a contraindication for them and as mentioned before ET was a limb-saving treatment for them. The mortality, in fact, was not a result of the procedure but it seemed to be a result of the patient’s bad cardiac status and it could have happened with higher chances if patients underwent surgery.
| Study | Lesion Type | Stent Type | Number of Cases | Primary Patency Rate | |
|---|---|---|---|---|---|
| Bare | Covered | ||||
| Lammer et al. (26), 2000 | TASCII B, C, D | + | 61 L | 91% / 12 months | |
| Ali et al. (27), 2003 | TASCII C, D | + | 22 L | 84% / 24 months | |
| Rzucidlo et al. (13), 2003 | TASCII B, C, D | + | 34 L | 70% / 12 months | |
| Wiesinger et al. (28), 2005 | TASCII A, B, C, D | + | 60 L | 90.7% / 12 months | |
| Chang et al. (14), 2008 | TASCII C, D | + | 193 L | 87% / 5 years | |
| Sabri et al. (15), 2010 | TASCII A, B, C, D | + | 26 L | 92 / 24 months | |
| Mwipatayi et al. (29), 2011 | TASCII B, C, D | + | 81 L | 92% / 18 months | |
| Bosiers et al. (30), 2013 | TASCII C, D | + | 91 L | 91.1% / 12 months | |
| Humphries et al. (16), 2014 | TASCII A, B, C, D | + | 64 L | 72% / 3 years | |
| Aihara et al. (2), 2014 | TASCII C, D | + | 190 P | 87% / 12 months | |
| Shen et al. (18), 2015 | TASCII D | + | + | 60 L | 93.6% / 12 months |
| Psacharopolo et al. (3), 2015 | TASCII D | + | 33 L | 90.9% / 24 months | |
| Grimme et al. (4), 2015 | TASCII B, C, D | + | 70 P | 90.2% / 6 month | |
| 49 P | 87.3% / 12 months | ||||
| Kasemi et al. (9), 2015 | TASCII D | + | + | 21 P | 95.2% / 12 months |
| 21 P | 90.5% / 3 years | ||||
| This study | TASCII D | + | 24 L | 92.3% / 3 months | |
| 22 L | 90.9% / 6 months | ||||
| 18 L | 83.3% / 12 months | ||||
Studies on Endovascular Treatment of Aortoiliac Occlusive Disease
In studies formerly performed, the 2-year patency rates reported varied between 58 and 95% (20-25). Aihara et al. reported an 87% patency rate for one year of follow-up that shows no significant difference with our result, although the complication rate in their study was 6.3%. Moreover, their patients suffered from different types of lesions with a dominancy of TASC II C and D, which can lead to better results due to complexity of type D lesions (10). Another study conducted by Shen et al. demonstrated a 93.6% patency rate for one-year post-endovascular therapy (11). The study population they used had a similar sexual and smoking distribution with our population; however, 53% of our patients had diabetes mellitus versus 28% in their study. It should be mentioned again that outcome differences could be a result of the fact that both studies involved a limited number of cases. Finally, in a study carried out by Grimme et al. using covered endovascular reconstruction of the aortic bifurcation (CERAB) technique to treat aortoiliac occlusive disease, they reported 90.2 and 87.3 % patency rates for 6 months and 1 year of follow-up, compared to 90.9% and 83.3%, respectively in our study (13). Other comparable results are major complications which were lower in their case [1.9% vs 6%] and technical success that was higher in our study [95% vs 100%]. Resembling most other studies, their lesions were not exclusively TASC II type D and some were type B and C that could similarly end in higher patency rates. Finally, Kasemi et al. used a different ET technique and stents for 22 patients (bare metal stents in kissing configuration in nine cases, covered stents in kissing configuration in nine patients and the aortic bifurcation reconstruction with the Y-guidewire configuration technique in four patients). He reported 95.2% at 1 year and 90.5% at 3 years as primary patency rates and states that different stent types and configurations used for the aortoiliac endovascular treatment offer all the benefits of these materials for treatment on a case-by-case basis. A comparison and list of studies on endovascular treatment for aortoiliac lesions can be found in Table 3.
One last topic that is of importance is to understand the factors that have an impact on the chance of re-occlusion of the lesion at the stent site. Although due to the limited number of cases in our study, a comparison could not be performed, other studies found interesting results that are attention worthy. Yilmaz et al., according to acquired data, believe that comparable results to surgery can be achieved in patients over 50 years of age who had an appropriate stent configuration and placement (30). However, Aihara et al. mentioning the latter study, states that they found that independent influencing factors are female gender and percentage of patent diameter in comparison to proximal aorta diameter after the procedure, not the stent type and configuration. Moreover, Aihara et al. brought to attention that their population of study was older patients and it might have caused the difference (2). Another study could not find a correlation between patency rate and diabetes mellitus, hypertension, hyperlipidemia, renal disease, coronary artery diseases or carotid disease among patients who underwent CRAB (3).
Patients suffering from aortoiliac occlusive disease and generally, peripheral arterial disease are older patients with a background of HLP, smoking and due to a similar pathophysiology, concomitant cardiac disease is prevalent among them (1). Open surgery has been shown to have a higher mortality risk in older individuals or those with congestive heart failure, ischemic heart disease, pulmonary insufficiency, ischemic ulcers or gangrene (31). Therefore, aortoiliac occlusive disease (AIOD) patients are at high risk for surgery. In addition, many patients have low life expectancy due to medical conditions especially cardiac diseases and the need for longer patency might lose importance which can introduce endovascular therapy as the first choice specifically in older patients with comorbidities. Even though primary patency rates can be lower in aortoiliac stenting compared to surgery (32), since re-intervention and further surgeries remain feasible, endovascular treatment could be considered valuable.
Endovascular therapy seems pretty attractive and safe since it contains a small number of complications most of which are minor and can be easily managed and the length of hospital stay is significantly lower. From another perspective, the non-invasive nature of procedure assures that re-intervention remains an option for occlusions (33). The latter is probably one of the reasons that secondary patency rates are higher than primary patency rates (3, 5, 8). Any other interventions for several purposes could be undertaken very soon. Of the issues that might make surgeons think twice when deciding to re-open a patient with a previous bypass surgery are adhesions in the abdomen and pelvis. This issue is minimal in endovascular therapy and could be counted as privilege. However, there is not much documented evidence concerning the issue, but it seems pretty obvious to most of the surgeons. Endovascular therapy also has better short-term economic results compared to surgery (4).
The small number of cases that were analyzed alongside not long follow-up can be considered as the limitations of this study. However, since the number of total aortoiliac occlusion cases is low, even small studies could be guiding. Furthermore, the nature of the study that did not involve control groups or open surgical groups is another factor. Finally, since different studies have been carried out in different population groups with different genetics and environmental backgrounds sometimes comparisons could be misleading.
In conclusion, according to the current study and other similar studies, it could be concluded that endovascular therapy is a viable treatment choice for patients suffering from total infra-renal bilateral aortoiliac occlusion lesions. Since fewer and less threatening complications are present, less morbidity and mortality and acceptable patency rate is expected, this treatment option can be specifically considered for high-risk patients medical status wise. Further and more thorough evaluations of the clinical outcomes, with clinical trials and cohort studies, are suggested.
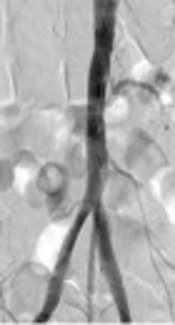
![A, Schematic view of the infra-renal bilateral aortoiliac occlusion [TASC II type D lesion]; B, Initial angiography shows infra-renal total aortoiliac occlusion [TASC II type D lesion]; C, Patient angiography after deployment of stents shows complete blood flow restored. A, Schematic view of the infra-renal bilateral aortoiliac occlusion [TASC II type D lesion]; B, Initial angiography shows infra-renal total aortoiliac occlusion [TASC II type D lesion]; C, Patient angiography after deployment of stents shows complete blood flow restored.](https://services.brieflands.com/cdn/serve/3170b/e159a95262db3078e8ec917b7069fc2f9a069713/iranjradiol-In_Press-In_Press-61843-g001-preview-preview.webp)