1. Background
Volumetric data acquisition and image processing have allowed for the accurate quantification of tumor volume (1, 2). The measurement of tumor volume in vivo has become an important factor in routine tumor evaluation and in the estimation of patient response to therapy (3) or in the planning of radiotherapy (4) in modern oncology. In cases of neuroblastoma, it is a greater treatment challenge when the individual is diagnosed with a massive intra - abdominal tumor. These tumors usually have a natural history that may be unalterable due to innate tumor biology, even if “optimal” or complete cytoreduction is achieved. The measurement of catecholamine metabolites such as vanillylmandelic acid (VMA) is also an important tool in clinical diagnosis as well as for the early detection of neuroblastoma (5, 6). In this study, measurement of the VMA level was applied to investigate the relationship between the initial computed tomography - measured primary tumor volume (CTPTV), the 24 - hour urine VMA level and the stage at diagnosis in patients with neuroblastoma before treatment.
2. Objectives
The purpose of this study was to investigate the relationship of initial computed tomography-measured primary tumor volume (CTPTV) with 24 - hour urine vanillylmandelic acid (VMA) level and stage in patients with neuroblastoma.
3. Patients and Methods
3.1. Patient Population
This retrospective study was approved by our institutional review board. We included patients with histologically confirmed neuroblastoma for whom initial 24 - hour urine VMA levels and computed tomography (CT) scans from January 2008 to December 2015 were available. Patients without initial 24 - hour urine VMA levels or CT scans were excluded. The medical charts of these patients were reviewed for demographic characteristics, tumor origins, and stage at diagnosis.
3.2. VMA and CT Imaging
We collected 24 - hour urine specimens to measure the VMA levels. Because the upper limit of urine VMA in the laboratory of our hospital is 9.8 mg/24 hours, we arbitrarily use this number as a cut - off value in comparisons. All intravenous contrast - enhanced CT examinations were obtained in the portal venous phase after administration of iodinated contrast material with a 64 - slice multi - detector CT scanner (SOMATOM Sensation 64, Siemens AG, Forchheim, Germany) at the primary tumor site. The volume computed tomography dose index (CTDIvol) ranged from 1 to 8 cGy. All images were reconstructed into axial and coronal sections with a 3 or 5 mm slice thickness and interval and were then sent to a picture archiving communication system (PACS) for interpretation. The time interval between the urine VMA examinations and CT scans was also recorded.
3.3. CT - Assisted Measurement of Tumor Volume
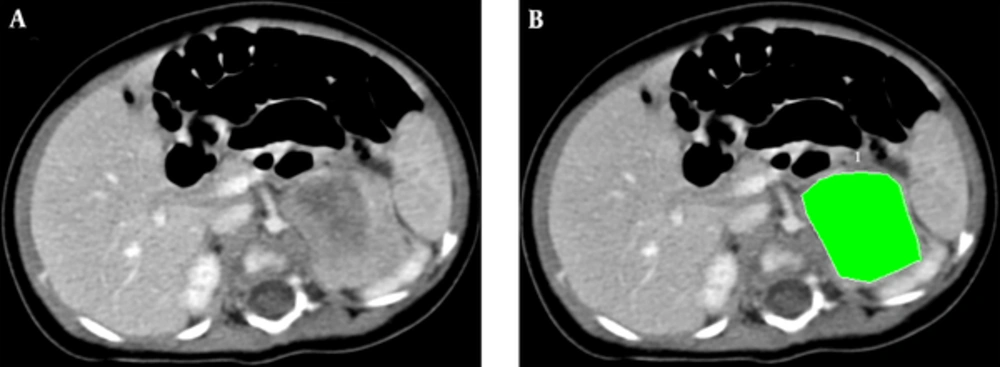
To establish the primary tumor volumes for this study, a board - certified radiologist who specializes in pediatric imaging manually traced the contour of the primary tumor with confluent lymph nodes, if present, on each axial CT slice at a dedicated working software “Syngo Workstation” (Siemens Medical Solutions, Erlangen, Germany). The images are displayed in an abdomen window setting (level = 60; width = 400) (Figure 1). Then, the tumor volumes were summed by multiplying the areas of the manually traced regions in each slice by the slice thickness (3 or 5 mm) and recorded. Intra - rater reliability was not performed.
3.4. Statistical Analysis
The results were obtained using descriptive statistics [frequency, percentage (%), mean, standard deviation (SD), median, and interquartile range (IQR)]. The correlation between the urine VMA levels and the CTPTV was assessed by the Pearson correlation coefficient. Further correlations stratified by age, VMA level, and staging were also conducted. To determine the differences in the CT tumor volume with respect to the demographic characteristics of the patients and the stage at diagnosis, the Mann - Whitney U test was applied for the analysis. Multivariate linear regression was used to estimate the effect of age, gender, urine VMA level, and stage at diagnosis for CTPTV. Besides, the correlation of VMA level with stage was assessed by the Pearson correlation coefficient and we conducted multivariate logistic regression model for stage based on sex, age, VMA as well. Moreover, a comparison of the demographic characteristics and the stage between CTPTV sizes (< 35 and ≥ 35 cm3) was performed using the Mann - Whitney U test, Chi - square test or the Fisher exact test. The statistical significance level was set as a P value of less than 0.05. The data were analyzed with SPSS 20.00 software (SPSS Inc., Chicago, IL, USA).
4. Results
Twenty - four patients (15 males and 9 females) were included in this study. All patients were diagnosed with neuroblastoma based on histopathology by surgery, tissue biopsy or bone marrow aspiration. The mean age at diagnosis was 2.62 ± 2.5 years. The mean VMA level was 33.51 ± 35.24 mg/24 hours. The mean CTPTV was 172.28 ± 264.85 cm3. The details are summarized in Table 1. The locations from which the tumors originated included the adrenal gland (N = 13 patients), retroperitoneum (N = 8), neck (N = 1), mediastinum (N = 1), and pelvis (N = 1). According to the international neuroblastoma staging system (INSS) criteria, the patients were classified into the following stages: stage 1 (N = 2, 8.3%), stage 2a (N = 2, 8.3%), stage 2b (N = 2, 8.3%), stage 3 (N = 1, 4.2%), stage 4 - S (N = 1, 4.2%) and stage 4 (N = 16, 66.7%).
| N (%) | |
|---|---|
| Age (years) | |
| Mean ± SD | 2.62 ± 2.5 |
| Median (IQR) | 1.7 (0.78 - 4.5) |
| Range | 0.02 - 8 |
| Age | |
| ≤ 1.5 years | 12 (50) |
| > 1.5 years | 12 (50) |
| Sex | |
| Female | 9 (37.5) |
| Male | 15 (62.5) |
| VMA (mg) | |
| Mean ± SD | 33.51 ± 35.24 |
| Median (IQR) | 20.05 (6.2 - 52.6) |
| Range | 1.6 - 124.1 |
| VMA | |
| ≤ 9.8 mg | 9 (37.5) |
| > 9.8 mg | 15 (62.5) |
| Stage | |
| Non - IV | 8 (33.3) |
| IV | 16 (66.7) |
| CTPTV (cm3) | |
| Mean ± SD | 172.28 ± 264.85 |
| Median (IQR) | 67.8 (23.55 - 171.25) |
| Range | 2.5 - 1021.2 |
Abbreviations: CTPTV, CT measured primary tumor volume; IQR, interquartile range; SD, standard deviation; VMA, vanillylmandelic acid.
The mean time interval between the urine VMA examination and the CT scan was 2.29 ± 2.61 days. The correlation coefficient between the urine VMA level and the CTPTV was analyzed in all patients, who were further stratified by age, gender, VMA level, and stage (Table 2). A moderate correlation was observed between the urine VMA level and the CTPTV in all patients (r = 0.673). When the patients were stratified by age, gender, VMA level, and stage at diagnosis, strong correlations were found in the groups of patients ≤ 1.5 years old (r = 0.739), in those > 1.5 years old (r = 0.732), and in patients with urine VMA levels greater than 9.8 mg (r = 0.769).
| Coefficient | P value | |
|---|---|---|
| All patients | 0.673 | < 0.001 |
| Age ≤ 1.5 years | 0.739 | 0.006 |
| Age > 1.5 years | 0.732 | 0.007 |
| Female | 0.356 | 0.350 |
| Male | 0.596 | 0.019 |
| VMA ≤ 9.8 mg | 0.378 | 0.316 |
| VMA > 9.8 mg | 0.769 | 0.001 |
| Non - stage IV | 0.230 | 0.585 |
| Stage IV | 0.592 | 0.016 |
Abbreviations: CTPTV, CT measured primary tumor volume; VMA, vanillylmandelic acid.
The comparison of the CTPTV between each subgroup (age, gender, urine VMA level, and stage at diagnosis) is summarized in Table 3. The median CTPTV of patients who were > 1.5 years old, who were male, those with a urine VMA level > 9.8 mg/24 hours and those with stage 4 at diagnosis were 95.5, 108.6, 113.8 and 118.1 cm3, respectively. Although the median CTPTV in patients who were > 1.5 years old was larger than that in patients who were ≤ 1.5 years old, this difference was not statistically significant. The median CTPTV in patients who were male was larger than that in patients who were female, this difference was not statistically significant. Among patients with a urine VMA level > 9.8 mg/24 hours, the median CTPTV was significantly larger than that in patients with a urine VMA level ≤ 9.8 mg/24 hours (P = 0.03). Additionally, among the patients who were diagnosed with stage 4 disease, the median CTPTV was significantly larger than that of patients who were diagnosed with non - stage 4 (P = 0.002).
| CT measured primary tumor volume (cm3) | P value | |||
|---|---|---|---|---|
| Mean ± SD | Median (IQR) | Range | ||
| Age (years) | 0.356 | |||
| ≤ 1.5 | 162.55 ± 259.06 | 24.95 (18.35 - 204.45) | 2.5 - 769.6 | |
| >1.5 | 182.01 ± 281.69 | 95.5 (48.95 - 144.75) | 7.3 - 1021.2 | |
| Sex | 0.073 | |||
| Female | 55.16 ± 66.62 | 24.1 (13.70 - 38.3) | 7.3 - 175.4 | |
| Male | 242.55 ± 314.00 | 108.6 (25.40 - 377.0) | 2.5 - 1021.2 | |
| VMA (mg) | 0.03 | |||
| ≤ 9.8 | 89.69 ± 196.31 | 24.1 (13.7 - 34.9) | 2.5 - 611.4 | |
| > 9.8 | 221.83 ± 293.6 | 113.8 (38.3 - 233.5) | 7.3 - 1021.2 | |
| Stage | 0.002 | |||
| Non - IV | 20.09 ± 9.98 | 23.55 (13.15 - 24.95) | 2.5 - 34.9 | |
| IV | 248.38 ± 298.3 | 118.1 (67.8 - 305.25) | 7.3 - 1021.2 | |
Abbreviations: CTPTV, CT measured primary tumor volume; IQR, interquartile range; SD, standard deviation; VMA, vanillylmandelic acid.
Multivariate linear regression model was conducted for CTPTV based on sex, age, VMA and stage. The result was summarized in Table 4. We found that the most effective variable was urine VMA level. When the VMA level increase every 1 mg/24 hours, the CTPTV will increase 4.85 cm3. The correlation coefficient between urine VMA and stage was 0.552, but multivariate regression model considering stage as a dependent variable and other variables as independent ones did not show a significant finding.
| Beta (95% confidence interval) | P value | |
|---|---|---|
| Age | - 8.31 (-47.12 - 30.51) | 0.675 |
| Sex | 115.95 (-47.53 - 279.43) | 0.164 |
| VMA | 4.85 (2.28 - 7.42) | 0.000 |
| Stage | 15.74 (-212.91 - 244.39) | 0.893 |
Abbreviations: CTPTV, CT measured primary tumor volume; VMA, vanillylmandelic acid.
The subgroup comparison of the demographic characteristics and stage at diagnosis between patients with CTPTV < 35 and ≥ 35 cm3 is summarized in Table 5. Among the patients with CTPTV ≥ 35 cm3, the median age (P = 0.011) and the median urine VMA level (P = 0.001) were significantly higher than those in patients with CTPTV < 35 cm3. Additionally, the 14 patients with CTPTV ≥ 35 cm3 were all stage 4 at diagnosis (P = 0.001).
| CTPTV | < 35 cm3 | ≥ 35 cm3 | P value |
|---|---|---|---|
| Age (years) | 0.011 | ||
| Mean ± SD | 1.47 ± 2.17 | 3.44 ± 2.46 | |
| Median (IQR) | 0.57 (0.16 - 1) | 3.00 (1.40-5) | |
| Range | 0.02 - 6 | 0.74 - 8 | |
| Sex | 0.092 | ||
| Female | 6 (60%) | 3 (21.4%) | |
| Male | 4 (40%) | 11 (78.6%) | |
| VMA (mg) | 0.001 | ||
| Mean ± SD | 10.04 ± 11.93 | 50.27 ± 37.05 | |
| Median(IQR) | 4.75 (1.9 - 12) | 42.8 (17.3 - 75) | |
| Range | 1.6 - 35.7 | 7.1 - 124.1 | |
| Stage | 0.001 | ||
| Non - IV | 8 (80%) | 0 (0%) | |
| IV | 2 (20%) | 14 (100%) |
Abbreviations: CTPTV, CT measured primary tumor volume; IQR, interquartile range; SD, standard deviation; VMA, vanillylmandelic acid.
5. Discussion
Neuroblastoma is a cancer that is derived from primordial neural crest cells, which are the precursors of the sympathetic nervous system and the adrenal medulla (7). Neuroblastoma is the most common extra - cranial solid childhood malignancy and is the third most common childhood tumor after leukemia and brain malignancy. It accounts for approximately 15% of childhood cancer deaths (8). The common sites of origin of neuroblastoma are the adrenal medulla (35% of cases), the extra - adrenal retroperitoneum (30% - 35%), and the posterior mediastinum (20%). Less common sites include the neck and the pelvis (9). The median age at diagnosis is 22 months. More than ninety percent of the patients are children aged less than 5 years, and the peak incidence occurs at 2 - 3 years of age. In addition, boys are more often affected than girls (10, 11). At least half of affected patients present with disseminated disease at the time of diagnosis (7). Among the patients in our study, the general characteristics of the patients were all compatible with those published in the literature.
In modern oncology, an accurate measurement of tumor volume in vivo has become an important clinical tool in the initial evaluation of tumors, radiotherapy planning and the assessment of patient response to therapy (12). Moreover, tumor volume had been regarded as a pretreatment prognostic factor in some other cancers (13-15). The urine VMA level is known to be elevated in patients with neuroblastoma due to defective catecholamine synthesis and metabolism. Thus, the VMA level has been considered as an evaluator and response indicator in neuroblastoma (5, 6, 16, 17). However, no previous studies have focused on the relationship between the 24 - hour urine VMA level and initial primary tumor volume in patients with neuroblastoma. In this study, we found a moderate positive correlation between the 24 - hour urine VMA level and the CTPTV in all groups of patients. This result was in agreement with our expectations. According to the subgroup analysis, the correlation coefficient was higher in the patient group with urine VMA levels > 9.8 mg than in patients with urine VMA levels ≤ 9.8 mg. This may have occurred because although the urine VMA levels were elevated in approximately 90 - 95% of neuroblastoma cases (18), approximately 5 - 10% patients presented with normal urine VMA levels regardless of the primary tumor sizes. Therefore, in the patient group with lower urine VMA levels, the correlation of urine VMA with the CTPTV is relatively weaker.
Age is a continuous variable in terms of prognosis, and it has been demonstrated that patients aged younger than 1.5 years are more likely to have a favorable outcome (11). It has been customary for clinical purposes to use 1.5 years of age as a cut - off point (19). In our study, although the median CTPTV in patients who were > 1.5 years of age was obviously larger than that of patients who were ≤ 1.5 years old, this difference was not statistically significant. The reasons for this lack of statistical significance may be the small case number and the extreme values in our study. However, we still perceived this tendency. After thorough analysis of the database, we chose a CTPTV of 35 cm3 as the cut - off point. In the subgroup analysis, the median age of the patients with an initial CTPTV ≥ 35 cm3 was significantly higher than that of patients with an initial CTPTV < 35 cm3. The average age of patients with CTPTV < 35 cm3 was 1.47 years, which was close to 1.5 years. This finding may imply that a smaller initial CTPTV (< 35 cm3) may be associated with a favorable outcome.
It is recognized that stage 4 disease at diagnosis is associated with a poor prognosis in patients with neuroblastoma. According to the subgroup analysis, the median CTPTV was statistically significantly larger in patients with stage 4 disease than in patients with nonstage 4 disease. This result was also in agreement with our expectations. The patients with advanced stage disease at diagnosis usually had a higher tumor burden. When 35 cm3 was used as the cut - off point for the CTPTV, we found that all fourteen patients with CTPTV ≥ 35 cm3 were diagnosed with stage 4. The positive predictive value was very high (100%), which suggests that a larger initial CTPTV (≥ 35 cm3) might be associated with an advanced tumor stage and that a larger initial CTPTV may indicate a poor clinical prognosis. However, no strong and direct evidence confirmed the relationship of initial primary tumor volume and clinical prognosis. A further study that focuses on this issue is therefore warranted.
Our study had several limitations. First, our study was retrospective, as a number of parameters could not be controlled for in a prospective study. Second, the case number was small, and statistical significance was difficult to achieve in this limited number of patients. Third, we could not evaluate the tumor volume within the bone marrow on CT scan, and we also did not calculate the tumor volume of the distal metastases. Thus, the actual tumor burden was likely underestimated. Finally, owing to lack of radiation, magnetic resonance imaging (MRI) has a competitive advantage over CT. However, MRI is not yet been used routinely in our children hospital in virtue of time consuming and risk of sedation. CT - assisted tumor volume measurement may be replaced by MRI shortly afterwards.
In conclusion, overall, a moderate positive quantitative correlation was observed between the initial CTPTV and 24 - hour urine VMA level in patients with neuroblastoma. The median CTPTVs were significantly larger in patients with urine VMA > 9.8 mg and in patients who were diagnosed with stage 4. The most effective variable for CTPTV was urine VMA level. When the urine VMA level increase every 1 mg/24 hours, the CTPTV will increase 4.85 cm3. The initial CTPTV ≥ 35 cm3 may be used as a cut - off point for the indication of advanced tumor stage. In the future, a larger trial that measures clinical outcomes is warranted to establish the association between the initial CTPTV and the prognosis of patients with neuroblastoma.