1. Introduction
Microglandular adenosis (MGA) is an uncommon glandular proliferation of the breast, which is most often a microscopic lesion but may present as a palpable mass. MGA is important because it consists of proliferation of small round glands in fibrous or fatty mammary stroma that mimics carcinoma clinically and pathologically (1). Breast carcinomas arising in MGA have been reported, and MGA is thought to be a precursor of breast carcinoma (2). We report a case of invasive carcinoma arising in MGA of the breast with multimodality imaging findings and review of the literature.
2. Case Presentation
A 63-year-old woman came to our hospital because of a palpable mass in the left breast. On physical examination, an approximately 2 cm mass was palpated in the upper inner quadrant of the left breast. Skin changes and nipple retraction were not found. No axillary lymphadenopathy was palpable. She had no personal or family history of breast cancer.
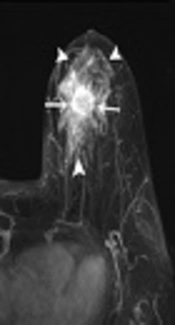
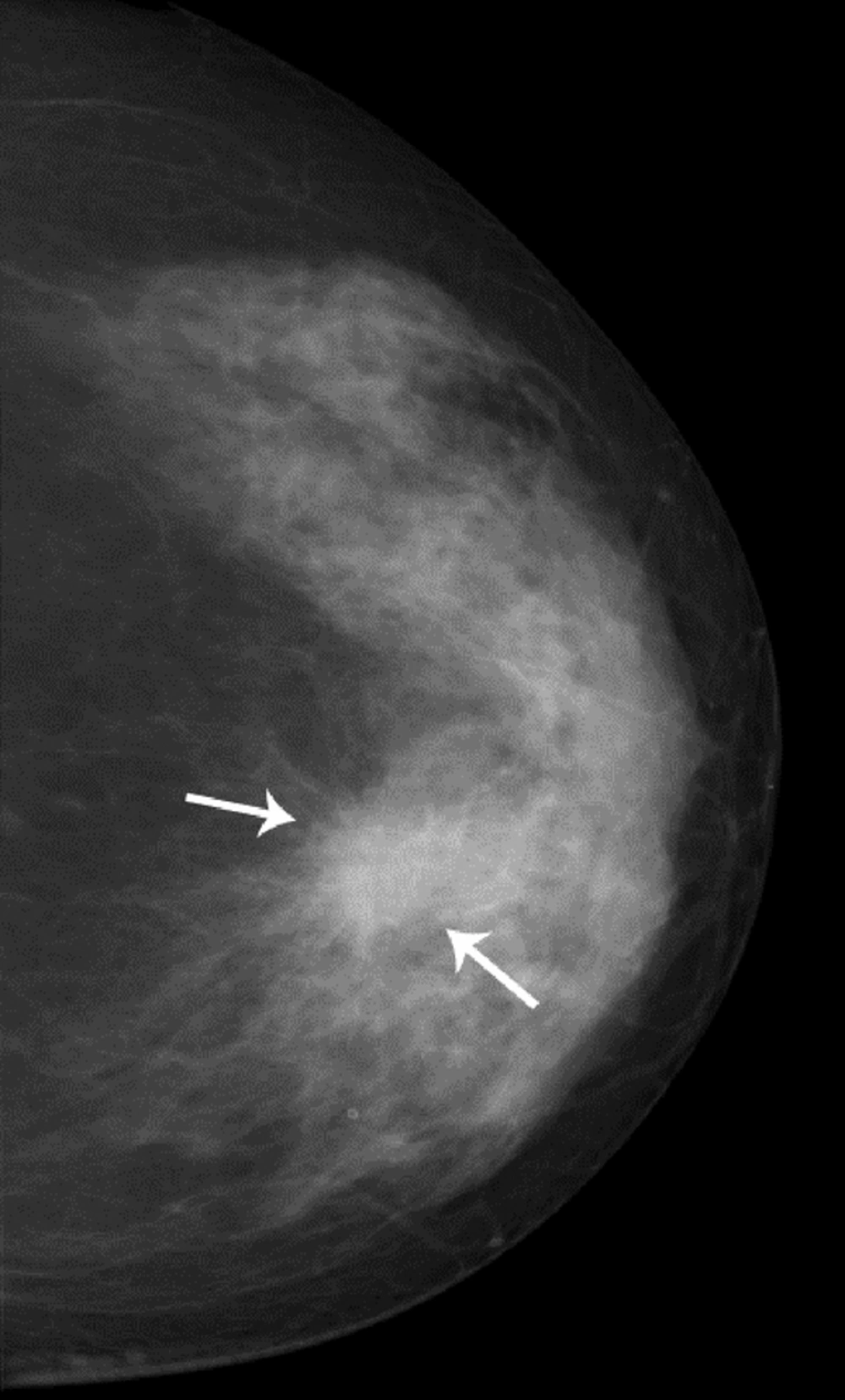
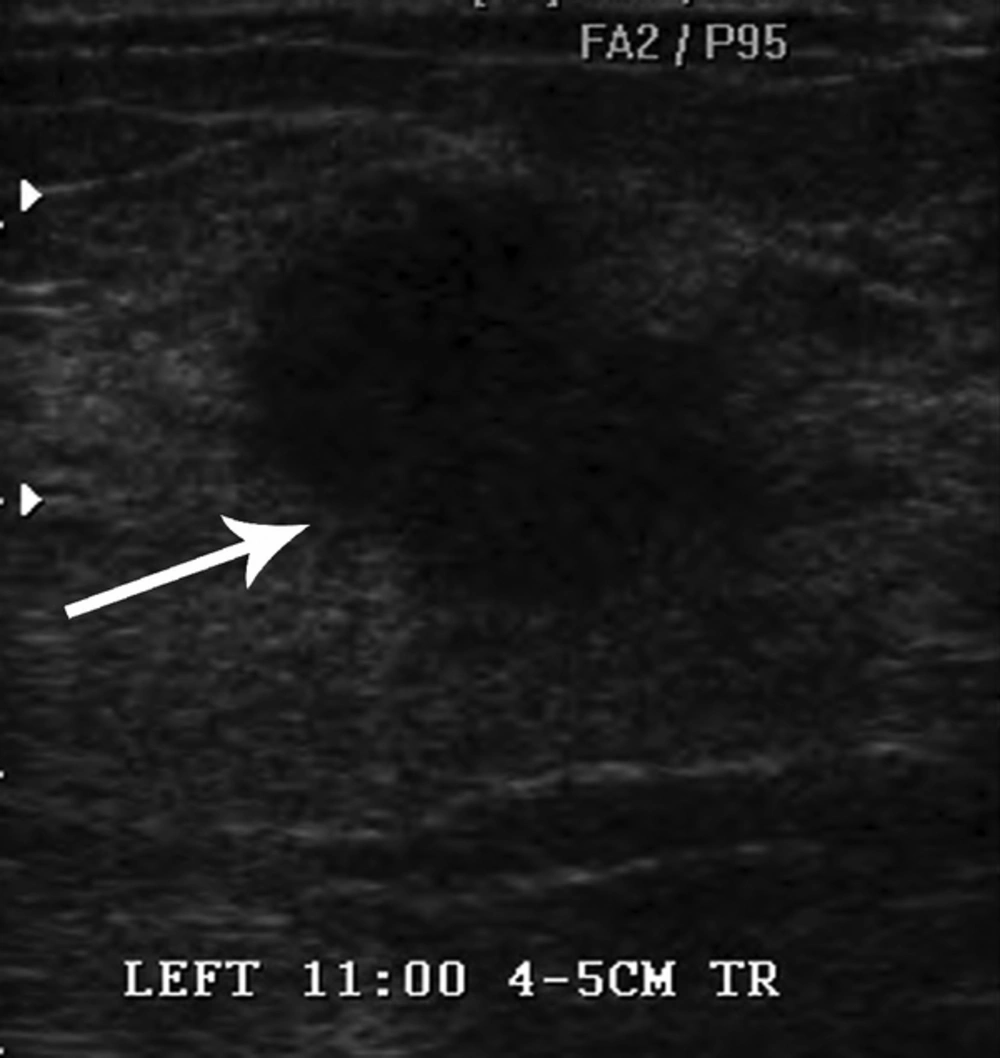
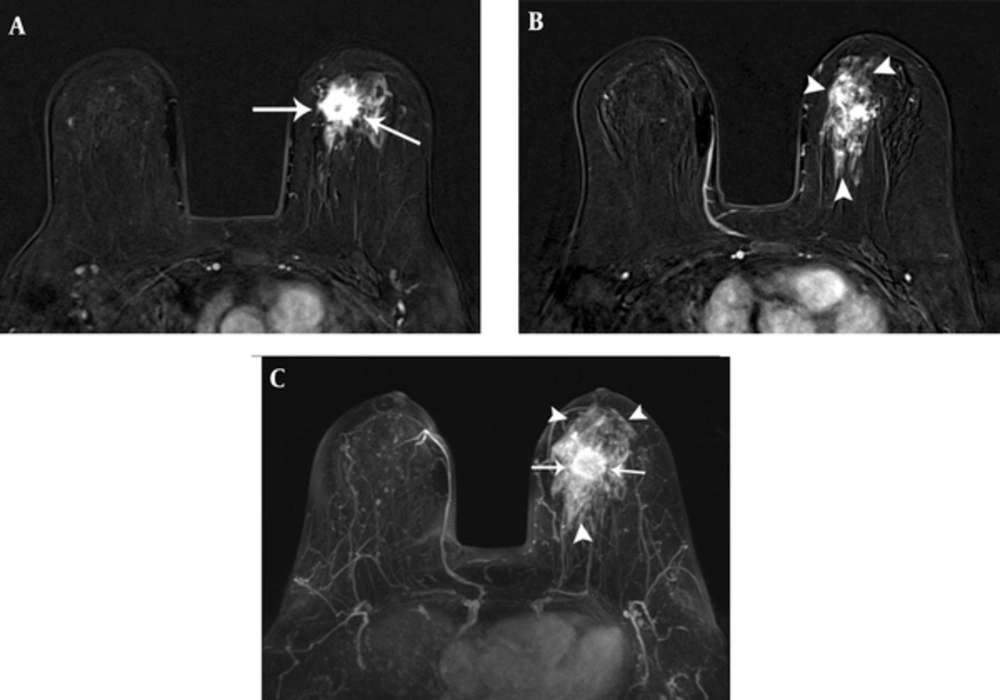
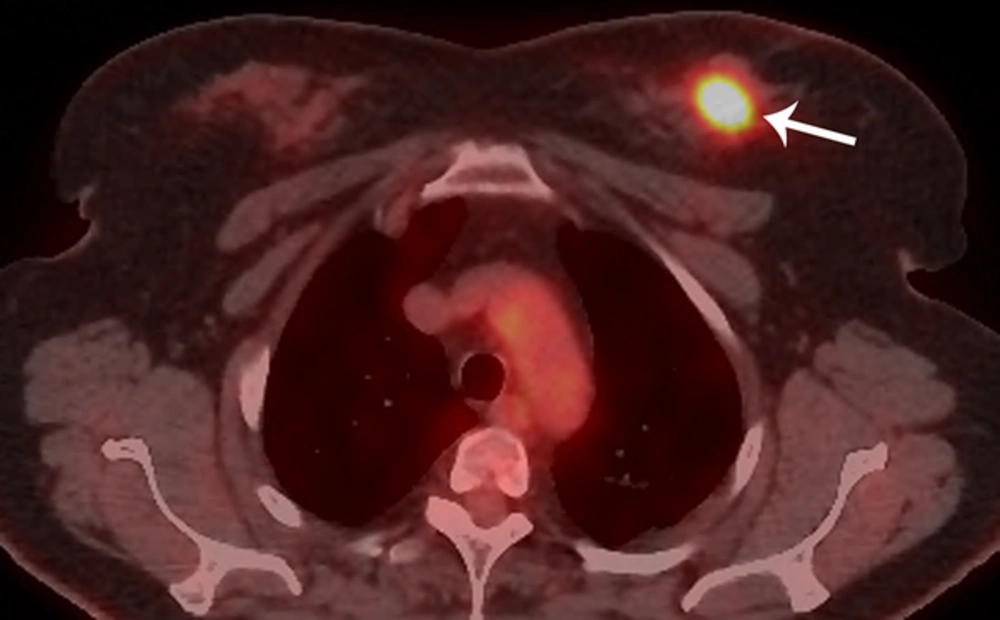
Bilateral mammogram showed a 2-cm irregular mass with spiculated margins in the 11 o’clock position of the left breast (Figure 1). No calcifications were seen. On sonographic examination, an approximately 2-cm, irregular, indistinct hypoechoic mass was detected (Figure 2). An ultrasound-guided core needle biopsy was carried out on the mass, which revealed invasive ductal carcinoma in the left breast. Breast MRI was performed for preoperative staging. MRI showed a 2.6-cm irregular mass in the upper inner quadrant of the left breast, which showed heterogeneous enhancement with a delayed washout kinetic pattern after administration of gadolinium. Additionally, segmental, heterogeneous non-mass enhancement surrounding the biopsy proven malignancy was seen in the upper inner quadrant of the left breast, which was considered a suspicious finding (Figure 3). Fluorine 18 (18F) fluorodeoxyglucose (FDG) positron emission tomography/computed tomography (PET/CT) demonstrated hypermetabolism (maximum standardized uptake value, 8.7) of the breast mass (Figure 4) without other abnormal hypermetabolism. Segmental mastectomy was planned. During surgery, the surgical margins were positive for ductal carcinoma on frozen section and modified radical mastectomy was performed. Sentinel lymph node biopsy was performed, which showed no evidence of metastasis.
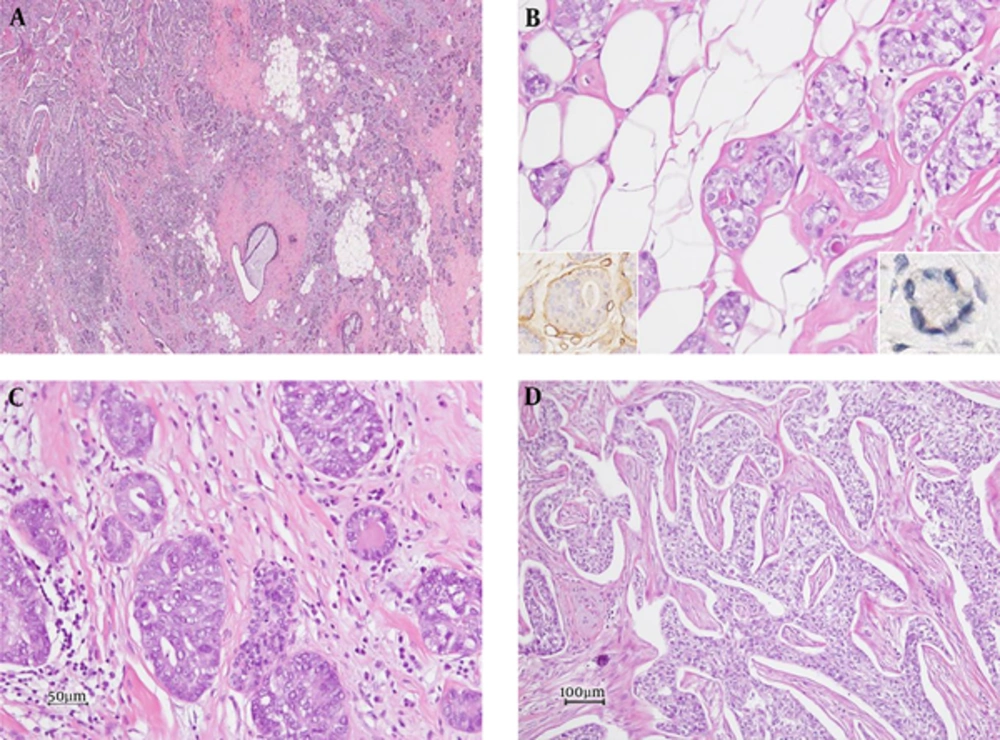
Grossly, a whitish-yellow colored, hard mass with ill-defined borders measuring 3.0 × 2.5 × 2.0 cm was noted in the upper inner quadrant. Microscopically, small glandular structures infiltrating fibrous stromal septa or fatty stroma were observed in the peripheral area of the mass (Figure 5A). The glands were lined with a single layer of epithelial cells with round nuclei and abundant vacuolated cytoplasm (Figure 5B). Eosinophilic secretory materials were seen in the lumen of the glands. Immunohistochemically, glandular structures were surrounded by laminin-positive basement membrane. No myoepithelial cells were demonstrable with immunohistochemical staining for smooth muscle myosin heavy chain and p63 (Figure 5B, inlet). The epithelial cells were positive for S100 protein but negative for smooth muscle actin, estrogen receptor, and progesterone receptor. These findings were consistent with MGA. In some regions, ductal carcinoma in situ was admixed with glands of MGA (Figure 5C). The main mass was an invasive carcinoma of no special type, modified Bloom Richardson grade 3 (Figure 5D). On immunohistochemistry, the carcinoma cells were negative for estrogen and progesterone receptors and HER2-neu. This case was finally diagnosed as invasive carcinoma arising in MGA.
Photomicrograph of the histopathological specimen. A, Microscopic examination shows a tumor with an ill-defined border (H&E staining, × 20). B, Microglandular adenosis composed of round glands lined by a single epithelial layer with laminin-positive basement membrane (left inlet, laminin immunohistochemistry) and lacking a myoepithelial layer (right inlet, p63 immunohistochemistry) are seen (H and E staining, × 200). C, Ductal carcinoma in situ admixed with microglandular adenosis is present (H and E staining, × 200). D, The main mass is an invasive carcinoma of no special type (H and E staining, × 100).
After surgery, six cycles of CMF (Cyclophosphamide, Methotrexate, and Fluorouracil)-based adjuvant chemotherapy were performed. After 32 months of follow-up, no recurrence was observed.
3. Discussion
MGA is an uncommon, benign breast disease considered to be a variant of adenosis. Diagnosis is frequently made by a pathologist, as the condition is often clinically asymptomatic. MGA is a rare epithelial lesion characterized by a proliferation of small round glands lined by a single layer of cuboidal epithelial cells with clear/vacuolated or eosinophilic cytoplasm and uniform nuclei (1). Unlike other intraductal proliferations and other forms of adenosis, the cells that line the glands do not have cytoplasmic protrusions or apical snouts, and myoepithelial cells are entirely absent, so the lesion may mimic well-differentiated breast carcinomas, including tubular carcinoma (2).
Although MGA is benign in its uncomplicated form, a spectrum of lesions, ranging from MGA to atypical MGA and breast carcinomas arising in MGA have been reported (3, 4). The presence of atypical MGA in areas of transition between MGA and carcinoma suggests that MGA increases the risk of developing carcinoma by serving as a precursor lesion (2).
Carcinoma arising in MGA has been reported in up to 27% of cases of MGA (1, 3, 5). Carcinoma arising in MGA may show both in situ and invasive components. The basement membrane, which is usually preserved around the glands of MGA and atypical MGA, tends to be disrupted in invasive carcinoma arising in MGA (6). No special type (NST) is the most common type of carcinoma arising in MGA. Rare cases of adenoid cystic carcinoma, carcinoma with secretory differentiation, squamous metaplasia, chondromyxoid metaplasia, basaloid features, or a mixture of NST and matrix-producing carcinoma have been described in association with MGA (3, 4). The immunohistochemical profiles of carcinomas arising in MGA are mostly of a triple-negative phenotype (i.e. lack of ER, PR and HER2) and express S100 and resemble to that of MGA (3).
Because of its rarity, the radiological findings of MGA are not well known and there have only been a few reports on imaging findings of MGA and carcinoma arising in MGA. Although mammogram may reveal localized increased density, MGA was not detected on mammogram in some studies (4, 7, 8). Sonography revealed an ill-defined low echoic lesion or hypoechoic mass with irregular borders, discrete microlobulations, and angular margins (7, 8). There has been only one previously published case report on MR imaging findings of MGA, and breast MRI showed a small non-circumscribed mass with moderate early and delayed enhancement (8).
Carcinomas arising in MGA can be detected as masses on mammography and sonography (4, 9, 10). Lee et al. reported that the lesion was an irregular shaped, hyperechoic nodule with indistinct margins and pleomorphic internal microcalcifications (9). In one study, the lesions were seen as irregular or lobular hypoechoic masses (10). In our case, mammogram and ultrasonography showed an irregular mass without calcifications. On breast MRI, not only an irregular mass but also segmental, non-mass enhancement surrounding the mass was evident. Pathologically, the irregular mass was confirmed as invasive carcinoma of no special type and most of the segmental, non-mass enhancement surrounding the mass was confirmed as MGA to atypical MGA. Although ductal carcinoma in situ was admixed with glands of MGA in some regions on histopathology, we could not differentiate ductal carcinoma in situ from MGA or atypical MGA on breast MRI. To our knowledge, this is the first case report of breast MRI findings that shows the wide spectrum of carcinoma arising in MGA.
When a core needle biopsy shows MGA, complete excision should be done and the excision specimens must be sampled thoroughly to achieve margins negative for the lesion and rule out the possibility of associated carcinoma. The treatment of carcinoma arising in MGA depends on the stage of the disease. It is important to assess the extent of the disease before surgery because MGA and atypical MGA may recur and carcinoma arising in MGA may occur if the affected area is incompletely excised (1, 4). Resetkova et al. reported a case of carcinoma arising in MGA that recurred 10 years after breast conservation surgery with incomplete resection of MGA, suggesting the importance of attaining complete excision to reduce the likelihood of recurrent carcinoma (5).
Breast MRI may be useful in assessing not only the extent of carcinoma but also the extent of MGA and atypical MGA, which is frequently occult on mammography. In our case, MGA and atypical MGA were seen as segmental non-mass enhancement surrounding the carcinoma. Various benign, high-risk, and malignant diseases in the breast can show non-mass enhancement on MRI. The differential diagnosis for non-mass enhancement on MRI includes pseudoangiomatous stromal hyperplasia, apocrine metaplasia, flat epithelial atypia, intraductal papilloma, radiation effect, atypical ductal hyperplasia, radial scar or complex sclerosing lesion, ductal carcinoma in situ, invasive ductal carcinoma, and invasive lobular carcinoma. Mastopathic changes such as adenosis, hormonal stimulation, inflammatory changes, and focal or diffuse fibrocystic changes are the most common benign causes of non-mass enhancement. Combined analysis of distribution, kinetics, and internal enhancement patterns of non-mass enhancement will facilitate better characterization of lesions (11, 12). More studies are needed to set up the characteristic MR imaging findings of MGA, atypical MGA and carcinomas arising in MGA, which can help in assessing the extent of the disease.
In summary, we report a case of invasive carcinoma arising in MGA of the breast. Although MGA is generally benign, complete excision should be considered to rule out the possibility of an associated carcinoma when a core needle biopsy shows MGA.