1. Background
Pulmonary embolism (PE) is a common, potentially life-threatening condition and one of the most frequent acute admissions for dyspnea and/or chest pain (1). A significant proportion of patients presenting with chest discomfort in the emergency department are referred to CT pulmonary angiography (CTPA) to rule out PE or even to “triple rule out” (PE, myocardial infarction, and aortic dissection) (2). Apart from chest pain (retrosternal or pleuritic), dyspnea, tachycardia, tachypnea and elevated D-dimer or decreased arterial partial oxygen pressure and/or hypercapnia raise clinical probability of PE (1, 3, 4). However, clinical symptoms may be vague, opening a broad differential diagnosis including pneumothorax, acute aortic or coronary syndromes, musculoskeletal and gastrointestinal disorders, and psychiatric conditions. Esophageal diseases are a common cause of chest discomfort and occur in more than one third of patients with excluded acute coronary syndrome (5). Daily reflux symptoms are reported in 4 to 7% of the population and about 2% suffer from esophagitis (6). Esophagitis as a relatively common disease, presenting as chest pain is often underdiagnosed. The majority of patients with esophagitis have abnormal findings on CT, especially esophageal wall thickening and abnormal enhancement pattern (target sign) (7).
2. Objectives
We hypothesized that CTPA could indicate esophageal cause of symptoms mimicking acute pulmonary embolism in some patients. To prove this, we retrospectively analyzed emergency CTPA scans to explore the signs of esophagitis and to estimate its frequency in patients with suspected PE.
3. Patients and Methods
3.1. Study Design
This retrospective study was performed in accordance with the declaration of Helsinki, it was approved by the local institutional review board, and the informed consent was waived. CT scans of patients, who were referred from the emergency department for CTPA between January 2013 and August 2014, were retrospectively evaluated by an experienced board-certified radiologist (>1000 CT angiography examinations) for the presence of PE and other chest pathologies.
3.2. CT Acquisition
The examinations were performed on either a 256-slice scanner (Brilliance iCT 256; Philips Healthcare, Best, The Netherlands) or a 16-slice scanner (Somatom Sensation; Siemens, Forchheim, Germany) with peak tube voltage of 100 kV or 120 kV respectively as breath-hold arterial phase acquisitions triggered by bolus tracking when attenuation in the pulmonary trunk exceeded 100 Hounsfield units (HU) and reconstructed in 3 mm sections.
3.3. CT Evaluation
Apart from evaluation of PE and other secondary findings, esophageal thickness and distention was measured at the distal end of the esophagus (at least 15 mm above the presumed level of the gastroesophageal junction), at the level of carina, and at the upper thoracic aperture. The measurement was done on axial images in the shorter diameter of the esophagus on its non-dependent side to avoid oblique measurement of the wall and intraluminal content that may not always be discernible from the esophageal wall. To assess intraobserver agreement, the distal esophageal thickness was later remeasured in 20% of the subjects.
An esophageal pathology was suspected, when the distal esophageal wall thickness was ≥ 5 mm regardless of luminal distension (7). For this purpose, we only considered the distal esophagus because of the nature of reflux esophagitis that extends in the oral direction from the gastroesophageal junction. If esophagitis was suspected, its extent was estimated. The occurrence of hiatal hernia and its size were recorded. The distal esophageal wall thickness was always measured above the hiatal hernia if suspected from the CT study. Finally, the angle of His was measured and patients with ample stomach content were identified.
3.4. Clinical Data
A board-certified specialist in gastroenterology (24 years’ experience) reviewed patients’ records for present complaints and endoscopy findings, where available. Each complaint was matched with possible explanations from the CT findings.
3.5. Statistical Analysis
Statistical tests were performed in Prism 5.0 (GraphPad Software, San Diego, USA) and SPSS 19 (IBM, Armonk, USA). To test for statistical significance, Mann-Whitney test or Fisher’s exact test were used in univariate analyses. Correlation between two values was expressed as Pearson’s correlation coefficient. Multivariate analysis was performed using binary logistic regression model. To assess intraobserver agreement we calculated intraclass correlation coefficient using two-way mixed model. A P value below 0.05 was considered significant.
4. Results
We included 434 consecutive patients, aged 67.5 ± 17.6 years, 46% patients were males. The most common symptoms reported by patients were dyspnea (67%), chest pain (38%), cough (25%), swelling of leg (24%), collapse (18%), fatigue or weakness (17%), and fever (13%). Twenty patients had undergone an operation during the previous two months, and 14 patients had deep venous thrombosis. CT confirmed PE in 27% patients. Secondary CT findings are listed in Table 1.
| Finding | May Explain Symptoms | The Only Explanation of Symptoms in Absence of PE | Total Number of Findings |
|---|---|---|---|
| Hiatal hernia | 39 (9) | 10 (2) | 138 (31.8) |
| Pleural effusion | 117 (27) | 4 (1) | 120 (27.6) |
| Pulmonary embolism (PE) | 119 (27) | x | 119 (27.4) |
| Esophagitis - 5 mm threshold | 52 (12) | 12 (3) | 87 (20.0) |
| Pulmonary emphysema | 35 (8) | 7 (2) | 59 (13.6) |
| Pneumonia | 51 (12) | 5 (1) | 53 (12.2) |
| Benign focal liver lesion | 0 (0) | 0 (0) | 45 (10.4) |
| Pulmonary edema | 42 (10) | 4 (1) | 44 (10.1) |
| Pulmonary nodule | 0 (0) | 0 (0) | 35 (8.1) |
| Pericardial effusion | 23 (5) | 1 (0) | 33 (7.6) |
| Pulmonary tumor or metastasis | 29 (7) | 1 (0) | 30 (6.9) |
| Thyroid nodule | 0 (0) | 0 (0) | 29 (6.7) |
| Malignant focal liver lesion | 4 (1) | 0 (0) | 21 (4.8) |
| Dilated ascending aorta | 5 (1) | 4 (1) | 16 (3.7) |
| Kidney cyst | 0 (0) | 0 (0) | 16 (3.7) |
| Coronary artery bypass grafting (CABG) | 4 (1) | 1 (0) | 11 (2.5) |
| Adrenal adenoma | 0 (0) | 0 (0) | 7 (1.6) |
| Aortic dissection | 6 (1) | 2 (0) | 6 (1.4) |
| Mediastinal lymphadenopathy | 5 (1) | 0 (0) | 5 (1.2) |
| Atelectasis | 5 (1) | 0 (0) | 5 (1.2) |
| Chronic thromboembolism | 5 (1) | 2 (0) | 5 (1.2) |
| Pulmonary fibrosis | 1 (0) | 1 (0) | 2 (0.5) |
Frequency of Findings in 434 CT Pulmonary Angiography Examinations in Emergency Patients and Their Ability to Account for Any of the Patients’ Symptoms or as the Only Explanation in the Absence of Pulmonary Embolism (PE)a
4.1. Esophageal Wall Thickness
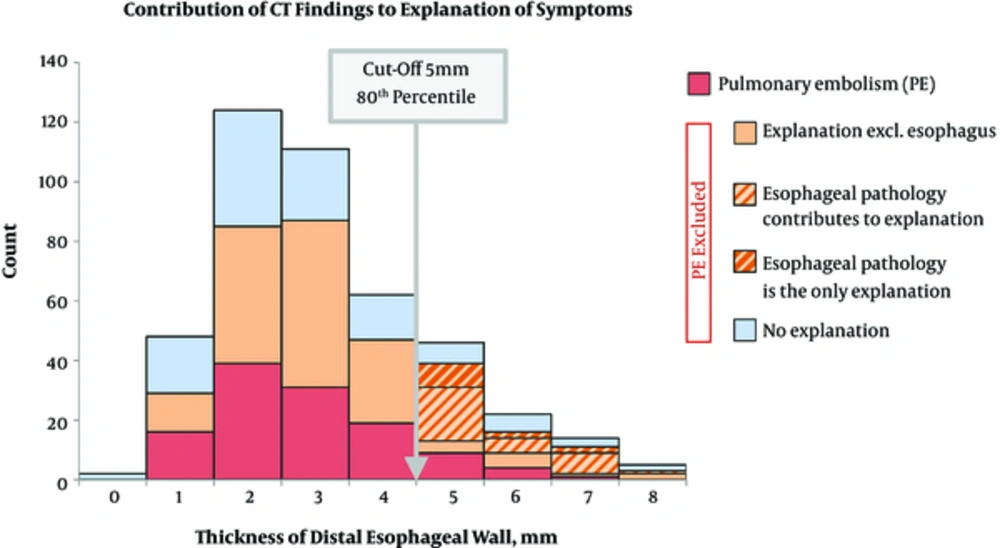
Average esophageal wall thickness was 3.6 ± 1.6 mm in the distal, 2.4 ± 1.1 mm in the middle, and 2.9 ± 1.1 mm in the proximal esophagus. In 87 patients (20%), the distal esophageal wall was thickened above the threshold of 5 mm (Figure 1). From these patients, PE was excluded in 87%, and 56% of them also had the symptoms consistent with esophageal disease (Figure 1). The esophageal wall thickness was indirectly and significantly related to the luminal distension (Pearson r = -0.31, 95% confidence interval (CI) -0.35 to -0.26, P < 0.0001). No significant correlation was found between the extension of esophageal wall thickening (107 ± 60 mm) and the distal esophageal wall thickness (6.1 ± 0.1 mm, where the thickness was ≥ 5 mm). Interestingly, the proportion of patients with thickened distal esophageal wall were balanced throughout the year, but there was fluctuation during the day with maximum in the afternoon. Hiatal hernia was identified in 32% of the patients (age 75 ± 11 years vs. 64 ± 19 years, P < 0.0001).
Contribution of CT findings to the explanation of symptoms. From the 434 patients, pulmonary embolism (PE) was found in 119 (red). In patients without PE, an alternative diagnosis explaining patients’ symptoms was established in 115 patients (orange) excluding 31, where esophageal pathology would be another alternative diagnosis (light orange diagonal), and 12, where it would be the only explanation of patients’ symptoms (dark orange diagonal). Orange color marks all patients, where any finding would explain at least one patient’s acute symptoms.
In 13% of patients with PE, the distal esophageal wall was thickened (≥ 5 mm) compared to 30% of patients without PE (P = 0.007). In the middle esophagus, the difference was 1% vs. 3% (P = 0.45) and in the upper esophagus 0% vs. 6% (P = 0.003).
No significant difference in the smoker status, alcohol intake, the presence or size of hiatal hernia, the angle of His, or stomach content between patients with normal and thickened distal esophageal wall was found. In patients with distal esophageal wall thickening, male gender slightly predominated (24% vs. 17%, P = 0.072) and the patients were slightly older (70 ± 16 years vs. 67 ± 19 years, P = 0.22).
The intraclass correlation coefficient for measurement of the distal esophageal wall thickness was 0.88 (P < 0.0001).
4.2. Correlation with Gastroscopy
Within 10 days from the CT examination, 42 patients underwent gastroscopy. Nineteen of them had thickened distal esophageal wall and from them 16 (84%) had finding of reflux esophagitis on gastroscopy with grade I (n = 6), grade II (n = 6), grade III (n = 3), and grade IV (n = 1) according to the Savary-Miller classification. From the 23 patients, who had normal thickness of the distal esophageal wall on CT, five (22%) had signs of esophagitis on gastroscopy grade I (n = 2), and grade II (n = 3, P = 0.0001).
4.3. Secondary Findings
Altogether, in 62% of patients without pulmonary embolism, secondary findings provided an explanation of their symptoms (Table 1).
4.4. Multivariate Analysis
Multivariate binary regression analysis model showed the following predictors of distal esophageal wall thickening: abdominal pain as a symptom (OR 4.7, 95% CI 1.5 to 14.8) and absence of pulmonary embolism (OR 2.4, 95% CI 1.1 to 4.9).
5. Discussion
Our study has found that 20% of acutely examined patients for suspect pulmonary embolism have CT signs of esophagitis. In the majority of them, this diagnosis would contribute to the explanation of symptoms.
CTPA is the gold standard in excluding PE (2, 8). Unlike scintigraphy, it may also reveal causes of chest pain and dyspnea other than PE (5, 9, 10). Gastroesophageal diseases, which are at the top of the list of differential diagnosis, are often overlooked because they mostly possess no imminent threat to the patient’s health (11). Thickening of esophageal wall is a sign of esophageal pathology, most commonly esophagitis, and esophageal spasm, and less commonly esophageal carcinoma and other diseases (7, 12, 13). Berkovich et al. found that patients with esophagitis had a mean wall thickness of 4.7 ± 2 mm, whereas healthy controls had a thickness of 2.9 ± 0.8 mm. They recommended using a 5 mm threshold and a target sign to differentiate between them, regardless of esophageal distension, because the lumen was mostly collapsed (7). In a well-distended esophagus achieved by hypotonia and ingestion of effervescent powder, the threshold for normal wall thickness can be decreased to 3 mm, because normal values range from 1.5 to 2.4 mm (mean 1.9 mm) (14). Even though there was a negative linear correlation between luminal distension and esophageal wall thickness, we decided to adopt the 5 mm threshold value, which is also the greatest from what has been proposed so far. We are aware of the fact that in reality and also in geometry, the relationship between distension and wall thickness is neither constant nor linear. Extensive correlation of endoscopic and CT findings was beyond the scope of this retrospective study. Nevertheless, endoscopic findings in the small number of subjects, who underwent upper endoscopy within 10 days from the CT examination, support our hypothesis.
In this study, the distal esophageal wall thickening ≥ 5 mm was encountered in 20% of all patients undergoing CTPA. In more than half of these patients with excluded PE, esophagitis would contribute to the explanation of their symptoms if it were adequately reported, instead of making a diagnosis of “non-cardiac chest pain”, or “excluded pulmonary embolism”.
The existence of esophageal disease was also supported by the finding that there were more patients with distal esophageal wall thickening who did not have PE and that the proportion of patients in whom esophagitis could be the only explanation of their symptoms increased beyond the 5 mm threshold value (Figure 1). Moreover, in patients with thickened distal esophageal wall, male gender slightly predominated, they were older, and their proportion increased steadily from the 6th decade upwards, which is consistent with the epidemiology of esophagitis (15). Additionally, the finding of thickened distal esophageal wall had delayed postprandial maximum during the day (afternoon), which is also typical for postprandial reflux.
The aforementioned facts indicate that a significant proportion of emergency patients undergoing CTPA have an esophageal pathology, most likely reflux esophagitis, because part of the examinations were performed due to acute chest discomfort among other complaints. We therefore advocate (and also practice), that at least in patients with no apparent cause of chest pain on CTPA, the thickness of distal esophagus should be measured and reported if it is 5 mm or more. Such patients may benefit from further diagnostic workup by means of endoscopy, pH monitoring, or at least a therapeutic trial with proton pump inhibitors may be attempted (16, 17).
There are several limitations of this study. First, oral contrast or effervescent powder was not given to enhance visualization of the esophageal wall, because it was not included into the standard preparation of patients undergoing CTPA. Second, the examination was performed in the arterial phase and therefore, we could not analyze abnormal enhancement pattern of the esophageal wall (target sign) as another indicator of esophagitis. Third, there were only 10% patients, who had gastroscopy within 10 days from the CT examination. This major limitation was to a certain extent substituted with pieces of indirect evidence supporting conclusions of this study. A tandem (same-day) upper endoscopy, pH monitoring, or fluoroscopy would be required to confirm the findings of this study. However, this would be difficult given the emergency context.
In conclusion, based on the findings in this study and review of the literature, we suggest that in emergency patients referred for CTPA to rule out PE or for “triple rule out”, the thickness of distal esophageal wall should be assessed if no other findings can explain the patient’s symptoms, and reported if it is at least 5 mm to identify a subset of patients, where esophageal pathology may have caused the symptoms.