1. Introduction
Leiomyosarcoma (LMS) is a malignant tumor that originates from mesenchymal tissue and most frequently happens in the uterus and gastrointestinal. Primary vascular leiomyosarcoma is particularly rare. Even so, about half of vascular origin LMS derive from the inferior vena cava (IVC) (1) that account for less than 0.5% of all soft tissue LMS (2). LMS has a female dominance (F:M ratio of 5:1) and mainly afflicts people aged 50 to 60 (3). Here, we report a case of LMS that was located in the suprahepatic IVC with intracardiac extension.
2. Case Presentation
A 46-year-old man was admitted to our hospital with an approximately one-month history of breathlessness that was persistent and got worse after activities. The patient denied other symptoms including fever, cough, sputum and chest pain. He denied any medical history of cardiovascular disease and pulmonary disease. His family history was insignificant. Physical examinations presented mild edema of the lower extremities. The laboratory values revealed tumor marker CA-125 of 242.6 U/mL, which was significantly elevated.
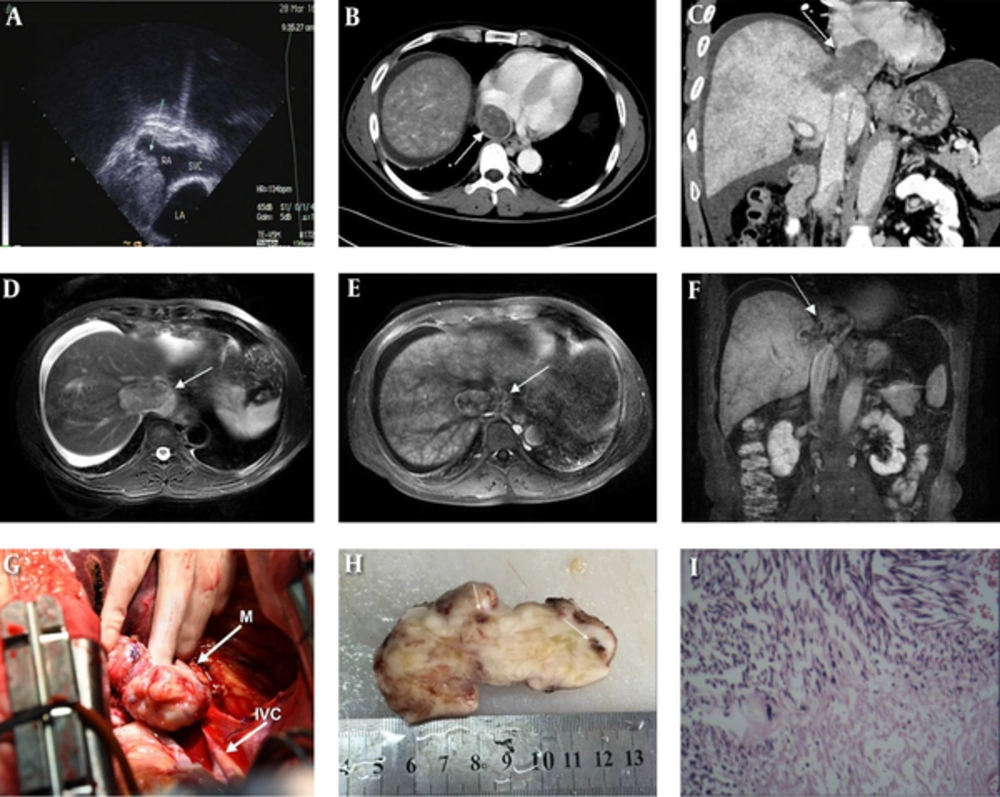
Transesophageal echocardiography demonstrated a non-mobile isoechoic mass occupying the IVC and the right atrium (RA) with unclear boundaries between the mass and the RA wall (Figure 1A). Transthoracic echocardiography revealed normal left ventricular systolic function with an ejection fraction of 56%. There was no evidence of adnexal mass on transabdominal ultrasound.
A 46-year-old man with leiomyosarcoma of the inferior vena cava. A, Transesophageal echocardiography demonstrates a non-mobile isoechoic mass occupying the right atrium (RA). B and C, Computed tomography venography shows a heterogeneous density mass with focal areas of relative low attenuation within RA (B, arrow) and reconstruction imaging shows an inferior vena cava (IVC) tumor extending superiorly into the RA and inferiorly to the hepatic parenchyma (C, arrow). D - F, Magnetic resonance imaging. Fat-suppressed T2-weighted image in the axial plane shows a relatively high signal intensity mass in the distal IVC involving adjacent hepatic parenchyma (D, arrow), and ascites is visible. The transverse and coronal postcontrast images show moderate persistent enhancement except for non-enhancement area in the central part (E and F, arrow). In addition, MR images show heterogeneous enhancement of the hepatic parenchyma. G, Intraoperative photo demonstrates the tumor originating from the IVC. H, Bisected gross specimen shows a solid tumor with spot hemorrhage (arrows), necrosis is unremarkable. I, Microexamination reveals that the tumor is composed of intersecting bundles of spindle-shaped cells. Hyaline degeneration is visible (H&E Stain, original magnification × 100).
Computed tomography venography of IVC was performed afterwards. Results showed a heterogeneous density mass that was located in the dilated distal IVC and extended superiorly into the RA and inferiorly to hepatic parenchyma. Meanwhile, the lesion appeared as inhomogeneous enhancement with some focal areas of relative low attenuation (Figure 1B). Reconstruction image marked the mass with a maximum length of 7.0 cm and width of 3.1 cm, and no abnormality was detected in other segments of the IVC (Figure 1C). Furthermore, the heterogeneous liver enhancement and ascites were the indirect signs of Budd-Chiari syndrome (BCS).
Magnetic resonance imaging (MRI) was carried out which also revealed a mass arising from the suprahepatic segment of the IVC with low signal intensity on T1-weighted image, relatively high signal intensity on T2-weighted image, as well as remarkably hyperintense signal on diffusion-weighted image respectively (Figure 1D). Dynamic contrast-enhanced presented with moderate heterogeneous enhancement in the arterial phase, duration enhancement in the portal vein phase and delay phase, and non-enhanced regions could be seen within the mass (Figure 1E - F).
Intraoperatively, the tumor was noted to originate from the junction of the hepatic veins and the IVC, infiltrating the right hepatic vein and the RA. The tumor was removed under extracorporeal circulation (Figure 1G). On gross examination, the mass was 8 × 5 × 3.5 cm in size. The cut surface of this solid tumor had a grayish-white, whorled appearance, with spot hemorrhage (Figure 1H). Histopathology revealed intersecting bundles of spindle-shaped cells with cytologic atypia, karyokinesis, and hyaline degeneration (Figure 1I). Immunohistochemistry proved the tumor was positive for vimentin, smooth muscle actin as well as desmin. Besides, CD34 and CD99 were partially positive. The proliferation marker of ki-67 was approximately 40%.
3. Discussion
Most of the IVC lesions develop secondary to original tumors elsewhere, including liver tumor, renal tumor, retroperitoneal tumor (4). Primary tumor of the IVC is extremely rare, whereas LMS is the most common. According to the segment of IVC involved, LMS can be divided into three types. Type I is below the renal veins. Type II is between the renal veins and the retrohepatic segment of IVC. Type III is located in the suprahepatic IVC. Type III is the most uncommon one of all cases (1). Cardiac extension is extremely less frequent and predominantly occurs at the superior segment. Fewer than one-third of the LMS grow within the lumen, while the remaining reveal a mainly extra-luminal growth (5).
Clinic manifestations which mainly correspond to its location appeared to be non-specific, including lower limb edema, abdomen pain, mass, back pain, renal hypertension, and dyspnea. Dyspnea is the most frequent symptom for Type III tumor that projected into the RA. Uncommonly, some cases presenting with BCS may result in congestive hepatomegaly, ascites, and jaundice. Radical surgery resection is the preferred method of treatment if possible. Adjuvant chemotherapy or radiotherapy can prolong survival in some extent. Despite this, patients still run a worsening prognosis, especially for these with suprahepatic tumors, IVC obstruction, and BCS (6).
Leiomyosarcoma is a slow-growing hypovascular tumor, sometimes hypervascular, corresponding to the involved segment and supplying arteries (7). Sonography may detect the tumor as a mixed hypoechogenic mass and show a dynamic view of intracardiac extension, but it has its limitations. Radiological techniques play an important role in the accurate diagnosis of LMS, particularly with CT and MRI. An irregular-shaped soft-tissue mass in the expending IVC accompanied with peripheral postcontrast enhancement and non-enhanced necrotic or cystic areas is the most common imaging finding (2, 8). Additionally, CT and MRI have good performance on some indirect findings of BCS such as intra and extra-hepatic collateral veins. Computer tomography venography (CTV) and contrast-enhanced MR venography (CE-MRV) allow to provide more details about both the location and the invasion of the tumor, give a comprehensive longitudinal view of the mass, whether it is predominantly confined to the lumen, or mainly extra-luminal growth, or has both components (9).
Generally, diagnosis of LMS could be made on the basis of tumor location, growth pattern, and imaging features. Nevertheless, LMS should be considered in the differential diagnosis of the following disorders: bland thrombus, tumor thrombus, intrahepatic neoplasm, primary retroperitoneal mass, intravenous leiomyomatosis (IVL), and primary leiomyoma of the IVC. The features of bland thrombus are well delineated filling defect within the lumen, with nonenhancement in general. Tumor thrombus develops secondarily to other neoplasm, and the enhancement pattern is similar to primary tumor. However, dilated IVC with venous wall or adjacent structures invasion by a heterogeneous enhancement mass might be related to the LMS. LMS of type II and/or type III, particularly the one with predominantly extra-luminal tumor growth should be identified with retroperitoneal mass. The sign of an imperceptible caval lumen at its point of largest touch with the mass is useful information that indicates that the origin of the tumor is IVC (10). However, retroperitoneal mass mainly present with obvious oppression of the contiguous cava. IVL is a benign tumor usually with a history of uterine fibrosis that behaves clinically in an aggressive way. A large continuous heterogeneous enhancing tumor growth along pelvic and systemic veins, IVC, finally extending into the heart, makes the most likely diagnosis of IVL (11), while leiomyomas usually appear as relatively localized growth and homogeneous enhancement without necrosis or cystic degeneration. In addition, tumors with intracardiac extension have to differentiated from primary or secondary cardiac tumors, such as myxomas. Typically, a mobile pedunculated mass movement with contraction and relaxation of the heart observed on echocardiography or cardiac magnetic resonance cine sequences is associated with myxomas.
The non-enhanced regions in this case are not in accordance with most of the literature that exhibit signs of necrosis or cystic degeneration. We therefore hypothesize that this kind of non-enhanced regions would be in connection with the insufficient delayed time after contrast injection, or owning to local hyaline degeneration inside the tumor. Besides that, we made another assumption, this is a primary leiomyoma of the IVC accompanied by foci of sarcomatous transformation that exhibited significant enhancement in peripheral areas.
3.1. In Conclusion
In our case, we described a patient with LMS of the suprahepatic IVC, which basically had intra-luminal growth and propagated to the RA. Radiological manifestation exhibited a soft-tissue mass characterized by heterogeneous postcontrast enhancement. CTV and CE-MRV were valuable in localizing the position and depicting anatomic relationship of the tumor to the surrounding structures which was crucial for guiding clinical management.