1. Background
Idiopathic granulomatous mastitis (IGM) is a rare and benign inflammatory breast disease of unknown etiology (1, 2). The clinical and radiologic findings of IGM may resemble those of breast carcinoma and can delay prompt treatment (3). IGM is usually noted during the reproductive age and in women who use oral contraceptives (4, 5). The disease is usually associated with recurrent attacks and may result in the formation of fistulas, ulceration, abscesses, erythema, and hardening of the skin (6).
Different management options may be implemented, and include conservative approaches with close surveillance, mastectomy, wide excision, abscess drainage, and immunosuppressive therapy with corticosteroids. However, there is no consensus on the optimal treatment of IGM (7, 8). Larger inflammatory masses are more frequently detected in patients with fistula and ulceration (9). Medication treatment may be unsuccessful in these patients and more invasive treatment methods such as therapeutic mammoplasty are recommended (10). However, surgical approaches may cause repeated surgical procedures, multiple scars, nipple and breast distortion (11).
2. Objectives
The purpose of this study was to ascertain if differences exist between fistular and non-fistular IGM with respect to clinical and radiological findings.
3. Patients and Methods
Fifty-two women with a histologic diagnosis of IGM were retrospectively investigated between February 2012 and December 2017. The study was approved by the local ethics committee and the need for informed consent was waived because of the retrospective design of the study. Other mastitis patients whose histopathologic diagnosis was not IGM, who previously underwent breast surgery, and/or who had a history of chemotherapy were excluded from the study.
All clinical evaluations were performed by a general surgeon with more than 14 years’ experience in breast surgery. Breast examination was performed for all the women in order to identify palpable lumps, nipple change, erythema, fistula, and ulceration.
Breast imaging findings were classified using the terminology described in the American College of Radiology Breast Imaging report and Data System (BI-RADS) lexicon 5th edition. Ultrasonography was performed using Aplio 500 (Toshiba Medical Systems Corporation, Tokyo, Japan) and a high-frequency (12 MHz) linear array transducer. Descriptive ultrasonographic characteristics of the lesions, including margin, shape, echo pattern, posterior acoustic features, and distribution were recorded.
Mammography (with Mammomat Inspiration, Siemens, Erlangen, Germany) was performed in all patients above the age of 40 years (n = 20). Mammography data in the craniocaudal and mediolateral oblique positions were obtained. Breast density pattern and morphological features were evaluated.
Magnetic Resonance Imaging (MRI) was performed using a 1.5 Tesla System (Aera, Siemens, Erlangen Germany). Axial plane images were taken with a breast coil; T2 weighted (T2W) (repetition time [TR]/echo time [TE]: 3960/105 ms); slice thickness, 3 mm, number of excitations (NEX), 1; matrix, 512 - 85; short tau inversion recovery (STIR) (TR/TE/inversion time [TI], 4120/99/160 ms); slice thickness, 3 mm; NEX, 2; and matrix, 320 - 85. Before and after intravenous contrast-injection 6-sequential fat-suppressed 3D T1-weighted images were obtained, and subtraction was performed. The contrast agent used was gadolinium-diethylenetriamine penta-acetic acid (DTPA) (0.1 mmol/kg; Omniscan), intravenously. The contrast enhancement patterns and skin thicknesses of the lesions were evaluated. Lesion areas were drawn manually in subtracted contrast-enhanced MR image and the lesion volume was determined by multiplying the areas by the interslice gap.
All radiological findings were reviewed on a workstation (Philips Extended Brilliance Workspace; Philips Healthcare) by a radiologist with 8 years of experience in breast radiology.
The histopathology of the 52 breast lesions was confirmed using an ultrasound-guided 14-gauge core-needle (Geotek Medical, Ankara, Turkey) biopsy. All histopathology results were evaluated by a pathologist with more than 12 years of experience in breast pathology.
3.1. Statistical Analysis
Statistical analyses were performed using SAS University Edition 9.4 (SAS Institute, Cary, NC, USA). Categorical variables are presented as frequencies and percentages. Fisher exact tests were used to compare proportions. The independent samples t-test was used to compare continuous variables. A P value < 0.05 was considered statistically significant.
4. Results
The patients’ mean age was 37.6 ± 7.4 (range 18 - 53) years. Of 52 IGM patients, fistula was detected in 19 (36.5 %), whereas no fistula was detected in 33 (63.4 %). None of the patients had a history of autoimmune disease, oral contraceptive use, or tuberculosis. All but three patients had a history of breastfeeding. At the time of presentation, two patients were in the lactation period.
The most common symptom was a palpable breast mass (86.5%). Erythema, nipple changes, and ulceration were more common in fistular IGM than in non-fistular IGM. Left-side (57.6%) involvement was more common than right-side (42.3%) involvement in all patients. Most of the patients were in the premenopausal period in both groups. The clinical findings, lesion characteristics and treatment outcomes of the patients are presented in Table 1.
| Fistula (+) n = 19 | Fistula (-) n = 33 | P value | |
|---|---|---|---|
| Patients | |||
| Age (range), y | 35.3 ± 6.1 (25 - 48) | 39 ± 7.8 (18 - 53) | 0.09 |
| Breastfeeding duration (range), mo | 25.9 ± 14.2 (3 - 60) | 20.9 ± 13.4 (0 - 48) | 0.21 |
| Premenopausal | 18 (34.6) | 29 (55.7) | 0.64 |
| Menopausal | 1 (1.9) | 4 (7.6) | |
| Symptoms | 0.02 | ||
| Palpable mass | 17 (32.6) | 28 (53.8) | |
| Breast pain | 15 (28.8) | 23 (44.2) | |
| Erythema | 8 (15.3) | 3 (5.7) | |
| Nipple change | 7 (13.4) | 2 (3.8) | |
| Ulceration | 7 (13.4) | 3 (5.7) | |
| Location of lesion | 0.09 | ||
| Right | 5 (9.6) | 17 (32.6) | |
| Left | 14 (26.9) | 16 (30.7) | |
| Lesion characteristics | |||
| Lesion volume (range), mm3 | 95 ± 68 (3.4 - 270) | 43.3 ± 54.9 (1.1 - 269) | 0.004 |
| Skin thickness (range), mm | 3.67 ± 1.07 (2.1 - 6.3) | 2.63 ± 0.73 (1.5 - 4.5) | 0.0001 |
| Unilateral axiliary adenopathy | 10 (19.2) | 12 (23) | 0.253 |
| Treatment outcomes | 0.21 | ||
| Steroid | 11 (21.1) | 25 (48) | |
| Steroid + methotrexate | 2 (3.8) | 4 (7.6) | |
| Steroid + bromocriptine | 0 (0) | 1 (1.9) | |
| Surgery | 6 (11.5) | 3 (5.7) |
Clinical Findings, Lesion Characteristics and Treatment Outcomes of Patients with Fistular and Non-Fistular Idiopathic Granulamotous Mastitis (IGM)a
Ultrasonography was performed in all patients and the most common finding was multiple irregularly shaped hypoechoic masses (44.2 %). Collection areas with complicated cysts consistent with abscess were commonly noted in patients with fistular IGM patients than in patients with non-fistular IGM. Twenty-two (42.3 %) patients had moderately enlarged axiliary nodes with mild cortical thickening and preservation of the hila. Mammography was performed in 20 patients and the main finding was focal asymmetry in 6 (11.5 %) patients (Figure 1). Heterogeneously dense breast patterns were noted in 15 (28.8 %) of the 20 patients. In one patient with fistular IGM, it presented with tissue distortion and global asymmetry. Calcifications and skin thickening were not present in any of the patients. The ultrasound and mammography findings in fistular and non-fistular IGM are summarized in Table 2.
| Fistula (+) n = 19 | Fistula (-) n = 33 | P value | |
|---|---|---|---|
| Ultrasonography findings | 0.025 | ||
| Multiple irregularly shaped hypoechoic masses | 7 (13.4) | 16 (30.7) | |
| Heterogeneous mass with indistinct borders | 2 (3.8) | 8 (15.3) | |
| Mass with circumscribed margin and posterior enhancement | 0 (0) | 3 (5.7) | |
| Collection areas with complicated cysts consistent with abscess | 9 (17.3) | 3 (5.7) | |
| Millimetric hypoechoic mass with indistinct borders | 1 (1.9) | 3 (5.7) | |
| Mammography findings | 1.0 | ||
| No mammography | 15 (29.2) | 17 (32.6) | |
| Normal | 1 (1.9) | 3 (5.7) | |
| Irregular focal mass | 1 (1.9) | 4 (7.6) | |
| Focal asymmetry | 1 (1.9) | 5 (9.6) | |
| Global asymmetry | 1 (1.9) | 4 (7.6) |
Ultrasonography and Mammographic Findings in Fistular and Non-Fistular IGMa
A 43-year-old woman with non-fistular idiopathic granulomatous mastitis of the right breast. A, Mammograms show focal asymmetric opacity; B, Subtracted contrast-enhanced magnetic resonance image shows a non-mass regional heterogeneously enhanced lesion; C, Core biopsy specimen demonstrates lobulo centric granuloma which contains neutrophils (black arrows) (Hematoxylin and Eosin [H&E] staining, 150×).
MRI was performed in all patients. The most common MRI enhancement pattern was non-mass lesions with regional heterogeneous enhancement (30.7 %). The most common MRI enhancement pattern was non-mass lesions with regional clustering ring enhancement pattern in IGM patients with fistulas (19.2 %), whereas, the most common MRI enhancement pattern in non-fistular IGM was non-mass lesions with regional heterogeneous enhancement (23 %) (Figure 2). Lesion volumes and skin thickness were higher in fistular IGM than in non-fistular IGM, with a statistically significant difference. In the kinetic analysis, the initial enhancement pattern of IGM was classified as slow in 36 (69.2 %) patients, medium in 13 (25 %) patients and fast in three (5.7 %) patients. In 34 (65.3 %) patients, the time-signal intensity curve revealed gradual and progressive enhancement (Type 1), and a plateau-like pattern following early contrast enhancement (Type 2) in 18 (34.6 %). No statistically significant differences were detected between the kinetic analysis findings of fistular and and non-fistular IGM patients. The MRI findings in fistular and non-fistular IGM are presented in Table 3.
| Fistula (+) n = 19 | Fistula (-) n = 33 | P value | |
|---|---|---|---|
| Mass-like lesion with irregular border and homogenous enhancement | 0 (0) | 1 (1.9) | 0.019 |
| Mass-like lesion with irregular border and heterogeneous enhancement | 2 (3.8) | 4 (7.6) | |
| Mass-like lesion with round shape and rim enhancement | 1 (1.9) | 7 (13.4) | |
| Non-mass lesions with regional clustering ring enhancement | 10 (19.2) | 3 (7.6) | |
| Non-mass lesions with diffuse heterogeneous enhancement | 2 (3.8) | 6 (9.6) | |
| Non-mass lesions with regional heterogeneous enhancement | 4 (7.6) | 12 (23) | |
| Initial phase | 0.883 | ||
| Slow | 14 (26.9) | 22 (42.3) | |
| Medium | 4 (7.6) | 9 (17.3) | |
| Fast | 1 (1.9) | 2 (3.8) | |
| Delayed phase (TSI curve) | 1.0 | ||
| Persistent | 12 (23) | 22 (42.3) | |
| Plateau | 7 (13.4) | 11 (21.1) | |
| Washout | 0 (0) | 0 (0) |
Magnetic Resonance Imaging Findings in Fistular and Non-Fistular IGMsupa
A 25-year-old woman with fistular idiopathic granulomatous mastitis of the right breast. A, Ultrasonography image shows a hypoechoic mass with tubular extension and fistula formation; B, Subtracted contrast-enhanced magnetic resonance image shows non-mass lesions with regional clustering ring enhancement and a fistula tract; C, Core biopsy specimen shows abscess formations (black arrows) (Hematoxylin and Eosin [H&E] staining, 100×).
Thirty six (69.2%) of the patients with fistular and non-fistular IGM were treated with only steroid (10 - 20 mg prednisolone) three times a day. The maximum duration of the steroid treatment was six months. Six (11.5%) of the patients with fistular and non-fistular IGM were treated with the combination of steriod and methotrexate (7.5 to 10 mg once a week) while only 1 (1.9%) patient with non-fistular IGM was treated with the combination of steriod and bromocriptine (5 - 10 mg daily). Nine (17.3%) of the patients with fistular and non-fistular IGM who did not respond to medical treatment underwent wide surgical excision. Surgical treatment in fistular IGM patients was performed more than in non-fistular IGM patients. Complications of steroid therapy did not occur in any of the patients. Post treatment follow up was performed with clinical examination, ultrasound and mammography between 3 and 6 months. No recurrences were detected in any of the patients.
5. Discussion
IGM is a rare and benign chronic inflammatory breast disease (12). Its etiology is not clear; however, local inflammatory response in the connective tissue due to the transition of the luminal secretions to the lobular breast stroma has been suggested as an etiological mechanism (13). The clinical and radiological findings of IGM usually mimic those of breast carcinoma, and the initial diagnosis is often breast carcinoma (14). Tissue sampling is still a commonly used method to differentiate these lesions (5). As the severity of the inflammation and the lesion size increase in patients with IGM, so does the frequency of ulceration and erosion in the breast tissue (9). Moreover, IGM manifests itself as different clinical and radiological findings in each patient (12, 15-17). In this study, we presented for the first time whether there are differences between radiological and clinical findings in fistular and non-fistular granulomatous mastitis.
Symptoms of IGM usually include a palpable mass and breast pain (18). Other symptoms such as erythema, ulceration, and nipple involvement are more infrequently detected (19). IGM more frequently presents as a unilateral breast mass; the involvement of both breasts is less common (20). The majority of patients with IGM are found in the premenopausal period and have a breastfeeding history (21). In our study, the most frequent symptom in IGM patients was a palpable mass and the second most common symptom was breast pain. Erythema, ulceration, and nipple changes were observed more often in IGM patients with fistulas, and most of the patients were in the premenopausal period.
Ultrasonography is the preferred imaging method for the evaluation of breast lesions. However, the ultrasound findings of IGM vary (16, 22). In their study, Yildiz et al. and Aghajanzadeh et al. detected multiple irregular hypoechoic masses and collections with tubular connections and with fingerlike aspects as the most common ultrasound finding (16, 17). Additionally, ultrasonography is useful for detecting fluid collections or abscess cavities, sinus tracts extending to the skin surface, and enlarged axiliary lymph nodes (2, 5). In our study, the most common finding in all IGM patients was multiple irregularly shaped hypoechoic masses (44.2%). Collection areas with complicated cysts consistent with abscess, were the most common ultrasound finding in fistular IGM, and a statistically significant difference was noted between fistular and non-fistular IGM regarding ultrasound findings. However, similar results were noted in both groups in terms of enlarged axillary lymph nodes.
The mammography findings of IGM are nonspecific (23). Because most of the patients were of reproductive age, mammography could not be applied to most of these patients, and the breast density of the patients who underwent mammography had decreased mammography sensitivity. In a study, Aghajanzadeh et al. identified an irregular focal mass as the most common mammography finding in 186 IGM patients (16). In another study by Yilmaz et al. the most common mammography finding was focal asymmetry (24). In our study, we performed mammography for only 20 patients, and the most common finding was focal asymmetry. However, there were no significant differences between the mammography findings in fistular and non-fistular IGM.
IGM has a wide range of MRI enhancement patterns such as intensively and strongly enhanced irregular masses, ring enhancement, regional or diffuse enhancement, and the formation of multiple abscesses with peripheral enhancement (5, 24, 25). In previous studies, the most common enhancement patterns on MRI were a regional area of non-mass enhancement and abscess with regional enhancement (15, 25, 26). In our study, the most common MRI enhancement pattern was non-mass lesions with regional heterogeneous enhancement. However, the most common MRI enhancement pattern in fistular IGM was non-mass lesions with regional clustering ring enhancement. Yet, statistically, the thickness of the skin and the volumes of lesions were significantly higher in fistular IGM than in non-fistular IGM.
This study had some limitations. The study design was retrospective and the sample size was relatively small. Moreover, inter- and intra-observer variability were not assessed in this study.
In conclusion, there were statistically significant differences between fistular and non-fistular IGM with respect to clinical and radiological findings. Imaging methods such as ultrasonography and MRI could be useful in identifying patients with a high risk of developing fistulas.
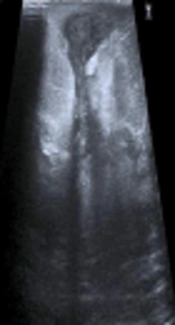
![A 43-year-old woman with non-fistular idiopathic granulomatous mastitis of the right breast. A, Mammograms show focal asymmetric opacity; B, Subtracted contrast-enhanced magnetic resonance image shows a non-mass regional heterogeneously enhanced lesion; C, Core biopsy specimen demonstrates lobulo centric granuloma which contains neutrophils (black arrows) (Hematoxylin and Eosin [H&E] staining, 150×). A 43-year-old woman with non-fistular idiopathic granulomatous mastitis of the right breast. A, Mammograms show focal asymmetric opacity; B, Subtracted contrast-enhanced magnetic resonance image shows a non-mass regional heterogeneously enhanced lesion; C, Core biopsy specimen demonstrates lobulo centric granuloma which contains neutrophils (black arrows) (Hematoxylin and Eosin [H&E] staining, 150×).](https://services.brieflands.com/cdn/serve/3170b/abb54ed08c044569632d736ad89ea521e41c9427/iranjradiol-79333-g001-F1-preview.webp)
![A 25-year-old woman with fistular idiopathic granulomatous mastitis of the right breast. A, Ultrasonography image shows a hypoechoic mass with tubular extension and fistula formation; B, Subtracted contrast-enhanced magnetic resonance image shows non-mass lesions with regional clustering ring enhancement and a fistula tract; C, Core biopsy specimen shows abscess formations (black arrows) (Hematoxylin and
Eosin [H&E] staining, 100×). A 25-year-old woman with fistular idiopathic granulomatous mastitis of the right breast. A, Ultrasonography image shows a hypoechoic mass with tubular extension and fistula formation; B, Subtracted contrast-enhanced magnetic resonance image shows non-mass lesions with regional clustering ring enhancement and a fistula tract; C, Core biopsy specimen shows abscess formations (black arrows) (Hematoxylin and
Eosin [H&E] staining, 100×).](https://services.brieflands.com/cdn/serve/3170b/fd1d3c9bf99caa5c43cfccb9f6b70beaae1ce0ff/iranjradiol-79333-g002-F2-preview.webp)