1. Background
Hemoglobinopathies, in particular thalassemia syndromes, are the most common monogenic disorders (1, 2). The carriers of thalassemia are estimated to be approximately 270 million worldwide (2). The number of affected new-borns is estimated to be annually approximately 60,000 (2), 23,329 of which are β-thalassemia major (3). Iran, which is located in the thalassemia belt, has various prevalences of thalassemia (1) for which the state government spent approximately $200 m in 2000 (4).
Iron overload (IO) is a major common complication of transfusion dependents and some of the transfusion non-dependent thalassemia variants. Conventionally, the mainstay of treatment in transfusion-dependent patients is blood transfusion to maintain haemoglobin above 9 g/L in adults (5). Poor growth, facial and bone abnormality and hemoglobin below 7 g/L are main indications leading to blood transfusion in children (6). Therefore, these patients are vulnerable to iatrogenic hemosiderosis due to blood transfusion. Moreover, upregulation of iron absorption from the intestinal tube and peripheral hemolysis expose both transfusion-dependent and non-dependent patients to IO (5-7).
Considering the fact that the liver is the major organ for storage of iron and that approximately 70% - 90% of iron is deposited in the liver parenchyma, the quantification of hepatic iron may represent a reliable alternative index for the total iron pool (8). Several methods have been presented to assess hepatic siderosis (HS). First, the direct assessment of liver iron using a needle biopsy has been accepted as the gold standard in evaluation of iron overload (5, 8). However, it is invasive and carries an approximately 5% complication rate (8) and is not easily acceptable to perform routinely in clinical practice. In addition, its accuracy is greatly affected by hepatocyte inflammation and the non-homogeneous distribution of iron throughout the liver (5). Second, serum ferritin has been widely used as a marker of iron storage. Although widely available, being inexpensive, well standardized and easily tolerated, it represents only 1% of the body iron pool (5). This method is not specific and its level is affected by inflammation, hepatocyte damage, vitamin C deficiency and oxidative stress (5, 9). Finally, magnetic resonance imaging (MRI) has recently been applied to quantitatively evaluate HS.
Two different MRI methods have been described in the literature for quantitative assessment of the amount of iron: spin echo sequences (SE) and gradient recalled echo sequences (GRE). Several available studies show that SEs (10, 11) and GREs (5, 11-13) are valid and accurate methods in the evaluation of hepatic iron. A study by Wood et al. shows that there is a linear relationship between the relaxometry in GRE and HS (11). In the clinical setting, GREs are more feasible, i.e., they are faster than SSEs and can be acquired during breath-hold in contrast to SSE sequences, which often get degraded by motion artefact. Although T2* (GRE) relaxometry is accurate, it is time consuming and not included in routine liver MR examination. Moreover, in patients with hepatic fibrosis, GRE is not reliable (14).
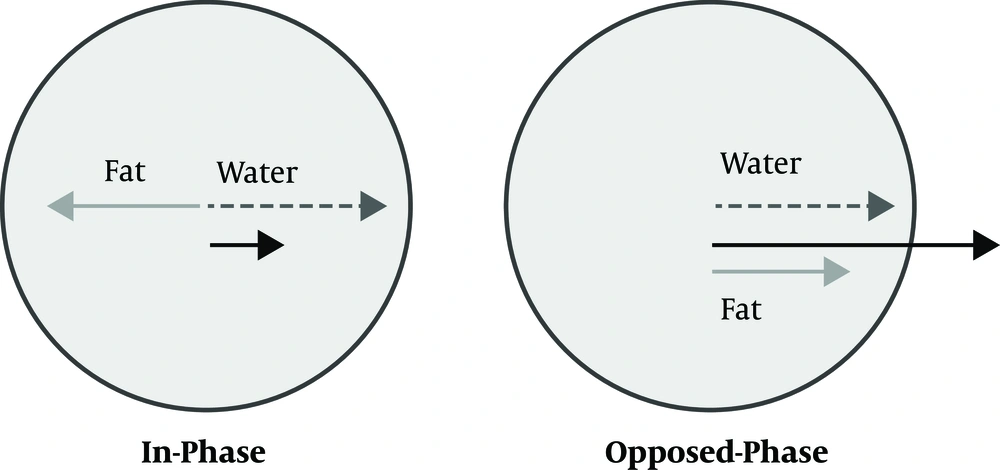
In spite of several studies regarding the accuracy of routine GREs and SEs to estimate the degree of hepatic siderosis, other methods are less assessed. One of such MR-based methods is chemical shift imaging (CSI). CSI is a well-established method for quantification of the amount of fat in the liver and is routinely included in most abdominal MR protocols nowadays. In some recent studies, the effect of IO on the estimation of steatohepatitis was assessed in animal and human models (15-17). In these studies, it is emphasized that iron is a confounding factor to assess the amount of fat. In fact, iron is the cause of signal drop in the chemical shift (CS) sequence and is more evident in IP with longer TEs than opposed-phase (OP). Therefore, if a statistically meaningful relationship could be detected between the amount of iron and signal loss in chemical shift imaging, CS imaging could be employed as a new method for assessment of the hepatic iron concentration.
Moreover, a water only sequence, a readily available sequence derived from main chemical shift sequences, may reduce the effect of fat signal and quantify the hepatic siderosis precisely. It may be an accurate alternative method for GRE sequences to reduce the charge, time and demand for further MRI sequences to quantify hepatic siderosis.
This concept has been tried and evaluated previously in three studies by Lim et al. (18), Virtanen et al. (19) and Schieda et al. (20); however, no consensus has been reached regarding the accuracy of CSI to assess HS and mainly because of lack of unified methods to minimize the confounding effect of fatty liver.
2. Objectives
The aim of our study was to assess the accuracy of CSI in comparison to relaxometry in GREs as a well-established method for the estimation of HS and to assess the accuracy of a water only sequence (WOS) to minimize the effect of fat on the estimation of hepatic siderosis.
3. Patients and Methods
3.1. Study Subjects
The study protocol was approved by the local ethics committee affiliated to our local institution (The ID is available). This is a prospective observational study in which 102 consecutive known cases of thalassemia major and intermedia, who attended our centre for iron assessment, were enrolled. After obtaining an informed consent, the patients underwent an MRI during a seven-month period from May to December 2016 in a tertiary referral centre.
3.2. MRI Scanner and Protocols
Studies were performed using a 1.5 T MR scanner (Avanto, Siemens Medical Solutions, Erlangen, Germany), using gradient strength of 35 mT/m.
All of the subjects underwent a single MRI protocol: a breath-hold T2* weighted image (GRE) by applying multiple TEs for relaxometry accompanied by a CSI obtained at the level of bifurcation of the portal vein.
To perform a dual-echo transverse two dimensional (2D) CSI (DIXON protocol), the following parameters were applied: repetition time (TR): 8 echo times (TE): 2 ms; opposed-phase (OP) and 5 ms: in phase (IP); Flip angle: 10º; slice thickness: 3 mm; matrix: 256*131; field of view: 352*264; and acquisition time: 12 sec.
An axial T2*-weighted gradient-echo sequence oriented perpendicular to the main portal vein with a superior saturation band was performed. The parameters were as follows: TR:193; TEs: 3, 6, 7, 11, 14, 18, 22, 26, 30, 34, 38, 42, 46; flip angle: 20º; slice thickness: 10; matrix: 128*102; field of view: 350*350; and acquisition time: 8 sec.
3.3. Image Analysis
The images with visible artefacts were excluded. To calculate the relative signal drop on CSI sequences, the following formulas were applied:
ISP = (Ir - Or)/Or
ISF = (IPS - OPS)/IPS
ISP: Iron signal drop percentage
ISF: Iron signal drop fraction
Ir: Signal intensity in in-phase sequence/signal intensity of paravertebral muscle in in-phase sequence
Or: Signal intensity in opposed-phase sequence/signal intensity of paravertebral muscle in opposed-phase sequence
IPS: Signal intensity in in-phase sequence
OPS: Signal intensity in opposed-phase sequence
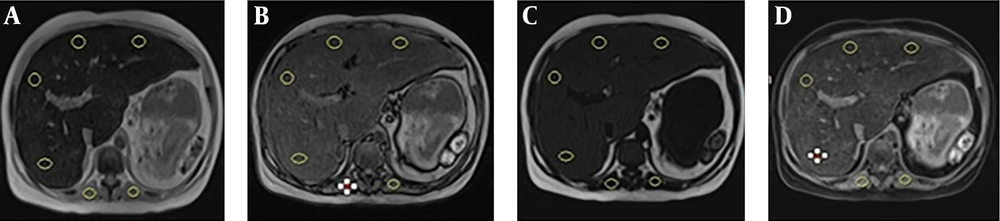
To assess the signal intensity, four 1 - 2 cm2 regions of interest (ROIs) were drawn on the liver at the level of bifurcation of the portal vein at the right anterior, right posterior, left medial and left lateral segments. The mean of signal intensity was the reference range to evaluate the effect of iron concentration in each sequence. In fact, the mean of signal intensity in IP, OP, WOS and fat only sequence (FOS) were compared with LIC. iron signal percentage (ISP) and iron signal fraction (ISF) were also calculated to quantify the signal drop in IP and OP sequences and compared with LIC, as well. All the ROIs were drawn by a single radiologist.
In addition, other ROIs were drawn on each paraspinal muscle in which macroscopic fat and areas of signal drop in and opposed phase image were avoided. The spleen was not used because of the possible iron deposition (Figure 1). This study was approved by the regional ethics committee.
To quantify the signal drop in the GRE sequences, relaxometry was conducted by implementing the previously mentioned parameters. ROIs were drawn in similar locations on the image at the level of bifurcation of the portal vein by using Segment Software V 1.8 (Segment: http://segment.heiberg.se), which was freely available for research purposes. The T2* value was calculated automatically and liver iron concentration was estimated according to the study by Wood et al. (8)
3.4. Statistical Analysis
Finally, the LIC was considered the reference standard, and the sensitivity, specificity, negative predictive value (NPV), and positive predictive value (PPV) were calculated. Receiver operating characteristics (ROC) curves were applied to determine the best cut-off points. Moreover, the correlations were assessed using the Pearson and Spearman rank correlation coefficient with a two-tailed P value, and scatter plots were drawn. The MedCal and Statistical Package for the Social Sciences program version 21.0 (SPSS Inc., Chicago, Il., USA) was used for the analysis. A P value of less than 0.05 was considered significant.
4. Results
One hundred and two patients were enrolled in this study, and approximately 77.5% were male with a mean age of 23.24 ± 8.7 years. The age ranged from 9 to 50 years. The majority of our research group comprised of thalassemia major subtype (70.4%) and the remaining 29.6% carried diagnosis of thalassemia intermedia subtype. Splenectomy had been conducted for 25.9% of these cases. The clinical and laboratory data are shown in Table 1.
| Variable | Mean ± SD |
|---|---|
| Duration of disease, y | 25.07 ± 8.57 |
| Time since splenectomy, y | 22 ± 11.4 |
| Time since first blood injection, y | 25 ± 8.6 |
| Time since first chelator administration, y | 22.08 ± 6.3 |
| Creatinine, mg/dL | 1.16 ± 1.4 |
| Calcium, mg/dL | 9.49 ± 0.9 |
| Ferritin, μg/L | 2636.86 ± 2656 |
| Triglyceride, mg/dL | 136.48 ± 81.6 |
| Cholesterol, mg/dL | 111.74 ± 28 |
| LDL, mg/dL | 53.95 ± 23.1 |
| HDL, mg/dL | 28.88 ± 9.4 |
| HB, g/dL | 9.38 ± 1.69 |
| AST, U/L | 32.96 ± 18.7 |
| ALT, U/L | 31.81 ± 23.7 |
Abbreviations; ALT, alanine aminotransferase; AST, aspartate aminotransferase; HDL, high density lipoprotein; HB, hemoglobin; LDL, low density lipoprotein; SD, standard deviation; y, year
ROIs were drawn on previously described sequences. The obtained values are shown in Table 2.
| Variable | Mean ± SD |
|---|---|
| Iron signal percentage | 38.54 ± 28 |
| Fat only signal intensity | 20.64 ± 11.7 |
| Water only signal intensity | 119.34 ± 92.1 |
| Liver iron concentration | 5.48 ± 2.6 |
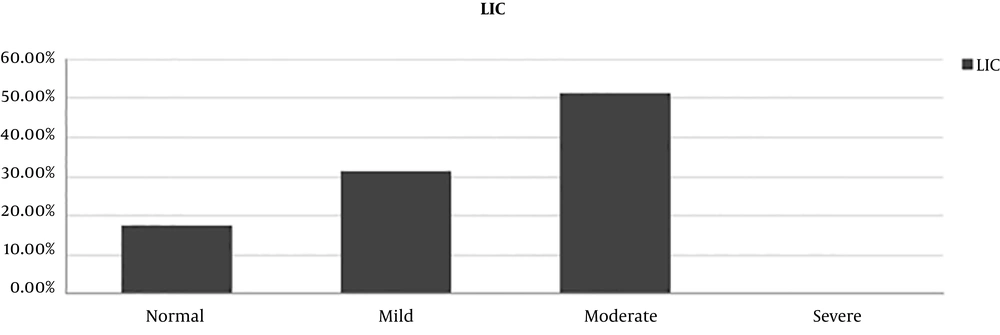
The distribution of the LIC estimated by T2* relaxometry is shown in Figure 2.
The correlation of LIC and signal drop in chemical shift sequences was evaluated using Pearson correlation (Table 3). Similarly, correlation coefficient was calculated for the other indicators of signal drop in CSI, including ISP and ISF. The correlation coefficient was 0.566 for ISP and 0.558 for ISF (P value < 0.005).
| Liver iron concentration | ||
|---|---|---|
| Correlation coefficient | P value | |
| IPSI | -0.698 | < 0.001 |
| OPSI | -0.590 | < 0.001 |
| FOSI | 0.365 | < 0.001 |
| WOSI | -0.640 | < 0.001 |
Abbreviations: FOSI, fat only signal intensity; IPSI, in-phase signal intensity; OPSI, opposed-phase signal intensity; WOSI, water only signal intensity
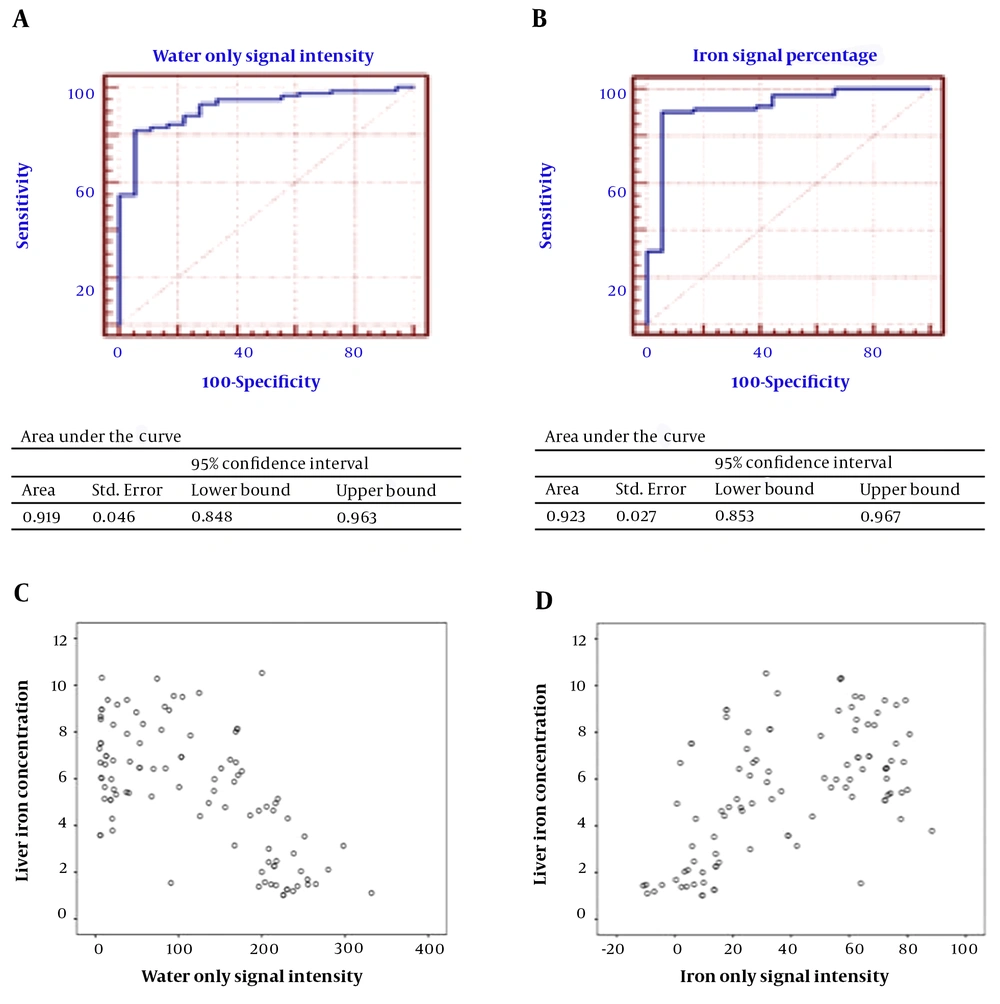
ROC curves were drawn (Figure 3), and the best cut-off points for sensitivity, specificity, positive predictive value and negative predictive value of ISP, ISF, in phase signal intensity (IPSI), out of phase signal intensity (OPSI) and fat only signal intensity (FOSI) were found and are shown in Table 4. While the LIC was the gold standard, the values lower than 40 μmol/g (2.2338 mg/g) were assumed to be in the normal range.
A, Receiver operating characteristic (ROC) curve and area under the curve (AUTC) of the iron signal percentage for diagnosis of liver iron concentration. B, ROC curve and AUTC of signal intensity in the water only sequence for diagnosis of liver iron concentration. C, Scatter plot of the liver iron concentration and signal intensity in water only sequence thalassemia patients. D, Scatter plot of the liver iron concentration and iron signal percentage.
| Liver iron concentration (prevalence of iron overload: 82.4%) | ||||||
|---|---|---|---|---|---|---|
| Cut-off point | Sens. (%) | Spec. (%) | NPV (%) | PPV (%) | AUTC (cm2) | |
| ISP | 13.6573 | 90.5 | 94.4 | 68 | 98.7 | 0.923 |
| 95% CI | 82.1 - 95.8 | 72.6 - 99.1 | ||||
| ISF | 0.0653 | 94 | 88.9 | 76.2 | 97.5 | 0.914 |
| 95% CI | 86.6 - 98 | 65.2 - 98.3 | ||||
| IPSI | 187.6775 | 88.1 | 94.4 | 63 | 98.7 | 0.941 |
| 95% CI | 79.2 - 94.1 | 72.6 - 99.1 | ||||
| OPSI | 219.21 | 86.9 | 83.3 | 57.7 | 96.1 | 0.899 |
| 95% CI | 77.8 - 99.3 | 58.6 - 96.2 | ||||
| FOSI | 11.6475 | 81 | 88.9 | 50 | 97.1 | 0.825 |
| 95% CI | 70.9 - 88.7 | 65.2 - 98.3 | ||||
| WOSI | 186.0075 | 82.1 | 94.4 | 53.1 | 98.6 | 0.919 |
| 95% CI | 72.3 - 89.6 | 72.6 - 99.1 | ||||
Abbreviations: AUTC, area under the curve; CI, confidence interval; FOSI, fat only signal intensity; IPSI, in-phase signal intensity; ISF, iron signal fraction; ISP, iron signal percentage; NPV, negative predictive value; OPSI, opposed-phase signal intensity; PPV, positive predictive value; Sens, sensitivity; Spec, specificity; WOSI, water only signal intensity
5. Discussion
Thalassemia major and intermedia are among the most prevalent monogenic disorders in Iran (1). Iron overload (IO) is a major complication of these situations, and the treatment is blood transfusion (5-7). Although several chelators have been introduced for the effective treatment of IO, the liberal administration of these agents is not warranted considering their associated serious side effects (21). An accurate IO estimation is a valuable means to reduce these effects. Liver biopsy and serum ferritin levels were previously the two major methods for assessment of iron overload, which are now considered invasive and somewhat inaccurate, respectively (5, 8, 9).
More recently, MRI-based methods have become popular to assess IO. Many centres across the world accept the GRE-based sequences and relaxometry as the main method for assessment of HS and the main indicator of total IO. Although relaxometry is accurate, it is time consuming and has certain limitations as previously described. In the present study, the accuracy of CSIs, which are almost always included in routine liver MRIs, is assessed. As mentioned above, iron deposition decreases the relaxation time in both IP and OP sequences. This signal drop is more evident in IP series with longer TEs than OP series.
The accuracy of IP and OP sequences to detect HS has been evaluated in three studies by Lim et al. (18) and Virtanen et al. (19) and more recently by Schieda et al. (20).
Controversial results are mainly evident because of the different methods to minimize the effect of fatty liver. Even a small amount of fat deposition results in a signal drop in OP when compared with IP. Theoretically, simultaneous deposition of fat and iron affects the signal intensity of IP and OP in different ways according to the relative amount of iron and fat. To minimize the effect of fatty liver, different strategies were applied in the previously mentioned studies.
The most acceptable method, which is tissue sampling, was applied by Lim. Unfortunately, it was not conducted for 29 out of 63 patients, and therefore, the effect was not completely evaluated. A visual scale was applied by Virtanen et al. In this semi-quantitative method, the signal intensity of liver in CSI was classified in comparison with muscle and background noise signal intensity by experienced observers. Scheida et al. questioned the visual method and applied fat signal fraction (FSF). Actually, they found that chemical shift signal intensity was correlated to LIC (CC = 0.65); however, after performing multivariate regression using FSF as an indicator of fat, the correlation was weakened and became insignificant (CC = 0.15),
However, FSF is not reliable because in the setting of moderate (most of our patients are in this category) and severe hepatic siderosis, the susceptibility effect of iron dominates the effect of fat deposition (22, 23). Moreover, our study reveals a significant correlation of FSF (FSF is similar to ISF in our study) with LIC, which supports the above-mentioned concept.
Besides, Lim et al. (18) used T2* SI rather than relaxometry as the indicator of hepatic siderosis. In this study, which was actually retrospective, different protocols and parameters were applied for the imaging of the liver. The sampling volume of this study was also limited.
For relaxometry in our study, 13 different TEs were applied. All patients were known cases of thalassemia major and intermedia and under observation and treatment for IO, which was potentially beneficial. As previously mentioned, very high estimated LIC above 300 μmol/g is not reliable (20). Therefore, it should be excluded as it was conducted in previous studies. Fortunately, no patient in our study had an estimated LIC above 300 μmol/g. On the other hand, hepatic siderosis was more prevalent in our study, which may justify the higher specificity and lower sensitivity of cut-off points in our series compared to the previous studies.
Our study showed that ISP could predict iron deposition, which emphasized the same findings of Lim et al. and Virtanen et al. (correlation coefficient = 0.566, P value < 0.001). The cut-off point was shown to be approximately 13.5%, similar to the cut-off point found by Virtanen (10%), with a lower specificity (94.4% vs. 100%) and higher sensitivity (90.5 vs. 85%). The positive predictive value (PPV) and negative predictive value (NPV) were 98.7% and 68%, respectively, with an area under the curve of approximately 0.923 cm2. In addition, ISF was shown to be a good predictor of HS.
An alternative method to diminish the effect of fatty liver is utilizing the water only sequence. As described before, the protons of fat and water are processed at different rates. By choosing the proper TE, the vectors of these protons come into phase and the signal intensity of the sum of these protons is shown in the IP sequence. Similarly, by choosing proper TE, their vectors become parallel in the opposite direction and the signal intensity is decreased and shown in the OP sequence (Figure 4). These two sequences can be combined mathematically in two different ways: water only (WO) and fat only (FO) sequences. In fact, the fat is suppressed in the Water Only sequences.
WO was shown to be an excellent sequence for the diagnosis of IO in our study (CC = -0.640, P value < 0.001). At the cut-off point of approximately 187, the specificity and PPV are 94.4 and 98.6, respectively. Sensitivity is 82.1, and the area under the curve is approximately 0.919 cm2. The FO sequence is not a reliable diagnostic tool for quantification of HS. Signal intensity in IP and OP was also shown to be correlated to LIC per se. However, clinical application seems to be limited because of lack of a reference signal and confounding factors affecting the signal intensity.
One limitation for our study was the lack of a gold standard in the evaluation of hepatic siderosis and hepatic steatosis. Invasive sampling is considered the gold standard but is no longer routinely utilized because of the potential complications and availability of highly accurate T2* techniques for quantification of hepatic siderosis. Missed medical record in a few cases was another limitation. However, no imaging or epidemiologic data, including signal intensities of different MR sequences, were missed, and therefore, the evaluation of the main concept of the authors, which is the efficacy of the WO sequence in the evaluation of HS, was unaffected.
In conclusion, chemical shift sequences are accurate enough for the detection of HS in thalassemia major and intermedia. The WO sequence is also a reliable method to minimize the effect of fatty liver and to detect HS.
.jpg)