1. Background
Angiodysplasia (AD), including arteriovenous malformation (AVM), Dieulafoy’s lesion (DL) and angioectasia (AE), is an important cause of gastrointestinal (GI) hemorrhage, especially for small intestinal bleeding (1). AD prevails in the terminal ileum, cecum and right colon (2). The main characteristics of AD include focal accumulation of tortuous and dilated microvascular anomalies within the mucosa and submucosa of the gastrointestinal tract, composing of thin-walled vasculature lined with endothelial cells with a bit of smooth muscle or not (3). The vasculature of AD is easy to break and thereby led to GI hemorrhage ranging from chronic, well-compensated anemia to acute lethal bleeding (4). The pathology and etiology of AD is not yet completely understood. The possible mechanism is that the frequently low-intensity obstruction in the venule of bowel wall resulting from smooth muscle contraction of GI tract increases the pressure in the capillary bed, which makes the anterior sphincter of capillary lose function and produces the arteriovenous fistula finally (5). AD may be present at birth, but may not become symptomatic until later in life. The patients of AD may present with recurrent episodes of melena, iron deficiency anemia and predominating anemia symptoms. The disease can progress with age and may deteriorate if not promptly treated.
2. Objectives
In the present study, we aimed to evaluate the effects of transcatheter arterial embolization (TAE) in the localization, diagnosis, and treatment of gastrointestinal bleeding due to AD.
3. Patients and Methods
3.1. Patients
This was a retrospective study. The study was conducted under approval of the Institutional Review Board. Informed consent was obtained from the patients. We reviewed the etiology, presentation, and management of patients with gastrointestinal hemorrhage due to AD identified on the basis of angiography between November 2002 and August 2018. The inclusion criteria included chronic or acute repeated gastrointestinal hemorrhage due to AD, failing conservative medicine treatment and endoscopic treatment; contraindication to surgery or refusal to surgery; and positive findings on the digital subtraction angiography (DSA). The exclusion criteria included very poor general conditions that were contraindications to angiography; more than three lesions or more than 5 cm of the bowel involved.
3.2. Angiography Technique
The procedures of transcatheter arterial embolization were performed by two interventional radiologists with more than 10 years of experience in their field. All patients underwent diagnostic radiography via femoral access. Angiography of the celiac trunk, gastroduodenal artery, left gastric artery, superior mesenteric artery, and inferior mesenteric artery were performed by 5-Fr RH catheters. A 2.2-Fr microcatheter (Terumo Medical Corporation, Tokyo, Japan; or Asahi intecc Co., Ltd., Aichi, Japan) was used for superselective catheterization of the target artery, if necessary. Coils (Cook Medical, Bloomington, IN, US) and poly vinyl alcohol particles (PVA, Alicon, Hangzhou, China) were used as embolic materials to block the arteriovenous shunts and the vessels responsible for the bleeding. Control angiography was performed once the embolization was completed. The procedure was stopped if the control angiography revealed that the abnormal vessels had disappeared or if there was no evidence of active hemorrhage.
3.3. Evaluation of Outcome and Follow-Up
After the embolization, blood tests were repeatedly performed to measure the hemoglobin level. The procedure was thought to be effective when the hemoglobin level was steady during the 3 days after the procedure, no symptoms or signs of hemorrhage were noted, and hemodynamic stability was achieved. Complications and follow-up details were recorded.
4. Results
In total, five males and seven females were included in the study. The mean age of the patients was 50 ± 18 years (range, 31 - 82 years). All the patients exhibited hemorrhage from the GI tract, including melena (n = 7), hematemesis (n = 2) and hematochezia (n = 5). Five patients had a history of repeated hematochezia or melena for more than ten years, who experienced persistent anemia, but did not exhibit any obvious symptoms. All the patients had no history of other diseases, including chronic hepatic disease, portal hypertension, inflammatory bowel disease (IBD), and extra peritoneal vascular disorders. Laboratory tests indicated decreased hemoglobin levels in all patients (mean = 7.6 ± 1.5 g/dL; range = 5.2 - 9.1 g/dL). Six patients were transfused with concentrated red blood cells (range, 2 - 8 U). Upper and lower GI endoscopy were performed in all patients within 12 hours after admission, and two bleeding lesions were observed in the stomach. Capsule endoscopy was performed in the patients with negative results of the upper and lower GI endoscopy examination, and flushing of local intestinal mucosa was observed in one patient. Ten patients underwent enhanced computed tomography (CT) prior to angiography, but no presence of contrast media was observed in the GI tract. Endoscopic treatments were performed in the patients with bleeding lesions in the stomach. Diluted adrenaline (1:10000 dilution) was injected into and around the bleeding lesion. In both patients, the endoscopic therapy failed, and the hemorrhage persisted. All the patients were not referred for surgery due to poor clinical conditions or refusal.
Embolization was completed in all patients, and no evidence of active bleeding was shown on the final angiographic run. Information on clinical presentations, embolized arteries, complications, embolic materials and follow-up details were presented in Table 1. The lesions included gastric AVM in two patients; ileal AVM in five patients; jejunal AVM in four patients; and AVM of ascending colon in one patient. PVA alone (150 - 350 µm or 350 - 560 µm in diameter) was used in nine patients; whereas, PVA in combination with coils were used in three patients. Positive findings on angiography included vascular tufts, broadening or narrowing of blood vessels, and a very early venous phase with/without extravasation of contrast media.
| Patient | Age, y | Complaint | Hb, g/dL | Location | Embolized artery | Diagnosis | Embolization agents | Complications | Three years follow-up outcome |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 31 | M and HM for 2 d | 8.9 | Stomach | Left gastric | AVM | PVA | No | No rebleeding |
| 2 | 33 | M and HM for 3 d | 7.4 | Stomach | Left gastric | AVM | PVA | No | No rebleeding |
| 3 | 52 | repeated M for 11 y | 5.2 | Ileum | Ileal | AVM | PVA | No | No rebleeding |
| 4 | 43 | HC for 2 d | 7.8 | Ileum | Ileal | AVM | PVA | No | No rebleeding |
| 5 | 76 | Repeated M for 24 y | 5.4 | Ileum | Ileal | AVM | PVA | No | No rebleeding |
| 6 | 75 | Repeated HC for 19 y | 7.2 | Ileum | Ileal | AVM | First session; PVA + coils; second session: PVA | No | Rebleeding 10 months later |
| 7 | 45 | M for one day | 9.1 | Ileum | Ileal | AVM | PVA | No | No rebleeding |
| 8 | 82 | Repeated M for 35 y | 8.6 | Jejunum | Jejunal | AVM | PVA and coils | No | No rebleeding |
| 9 | 32 | HC for one day | 8.9 | Jejunum | Jejunal | AVM | First session; PVA; second session: PVA + coils | No | Rebleeding 6 months later |
| 10 | 41 | HC for 5 d | 7.9 | Jejunum | Jejunal | AVM | PVA | No | No rebleeding |
| 11 | 53 | Repeated HC for 17 y | 8.8 | Jejunum | Jejunal | AVM | PVA | No | No rebleeding |
| 12 | 35 | M for 4 d | 5.5 | AC | Ascending colonic | AVM | PVA | No | No rebleeding |
Abbreviations: AC, ascending colon; AVM, arteriovenous malformation; Hb, hemoglobin; HC, hematochezia; HM, hematemesis; M, melena; PVA, poly vinyl alcohol particles.
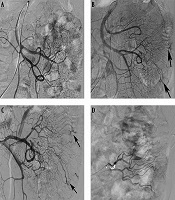
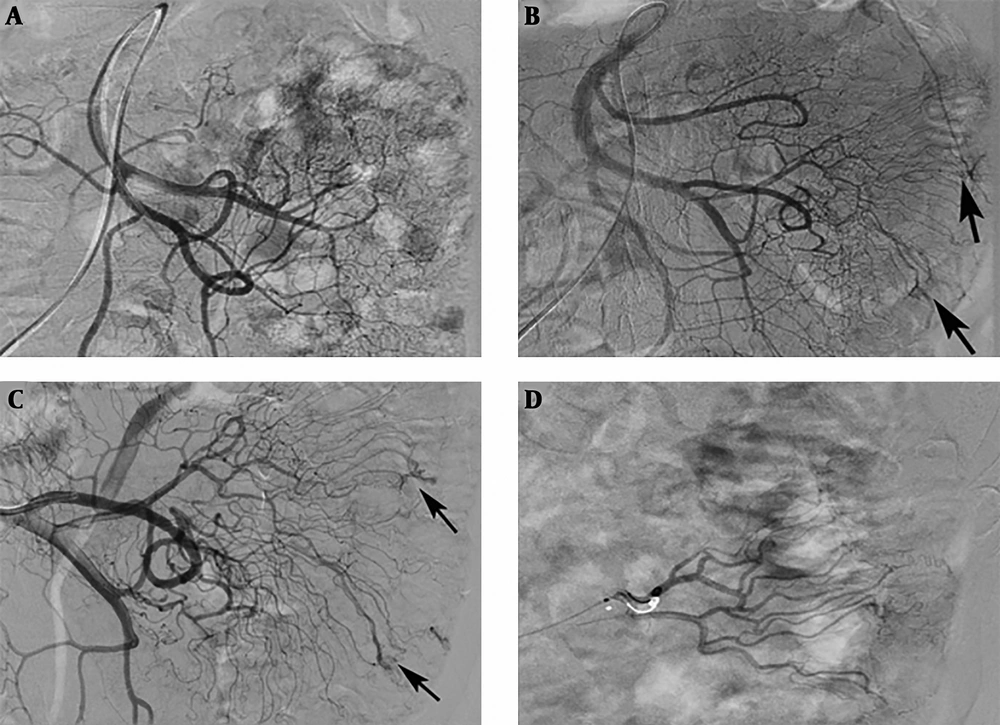
In three patients with repeated melena and chronic anemia, no positive findings were observed during the first angiography. However, repeated angiography indicated the lesions after 1 week (Figure 1). Two of these patients were finally diagnosed as ileal AVM and the other was diagnosed as colonic AVM.
An 82-year-old patient with a jejunal angiodysplasia (AD). A, No positive findings were observed on the first angiography; B and C, After 1 week, repeated angiography indicated abnormal vascular tufts and an early venous phase at the distal branch of the jejunal artery (arrows); D, Polyvinyl alcohol (PVA) particles (350 - 560 µm) and microcoils were used to embolize the abnormal blood vessels.
All patients were discharged without any severe complications. No rebleeding occurred within 30 days. In two patients with ileal AVM who underwent PVA injection alone (350 - 560 µm) during the first embolization, the hemorrhage recurred 2 years after discharge from the hospital. Both these patients underwent embolization once again, wherein PVA (350 - 560 µm) and microcoils were delivered to the abnormal vessels, and the bleeding stopped.
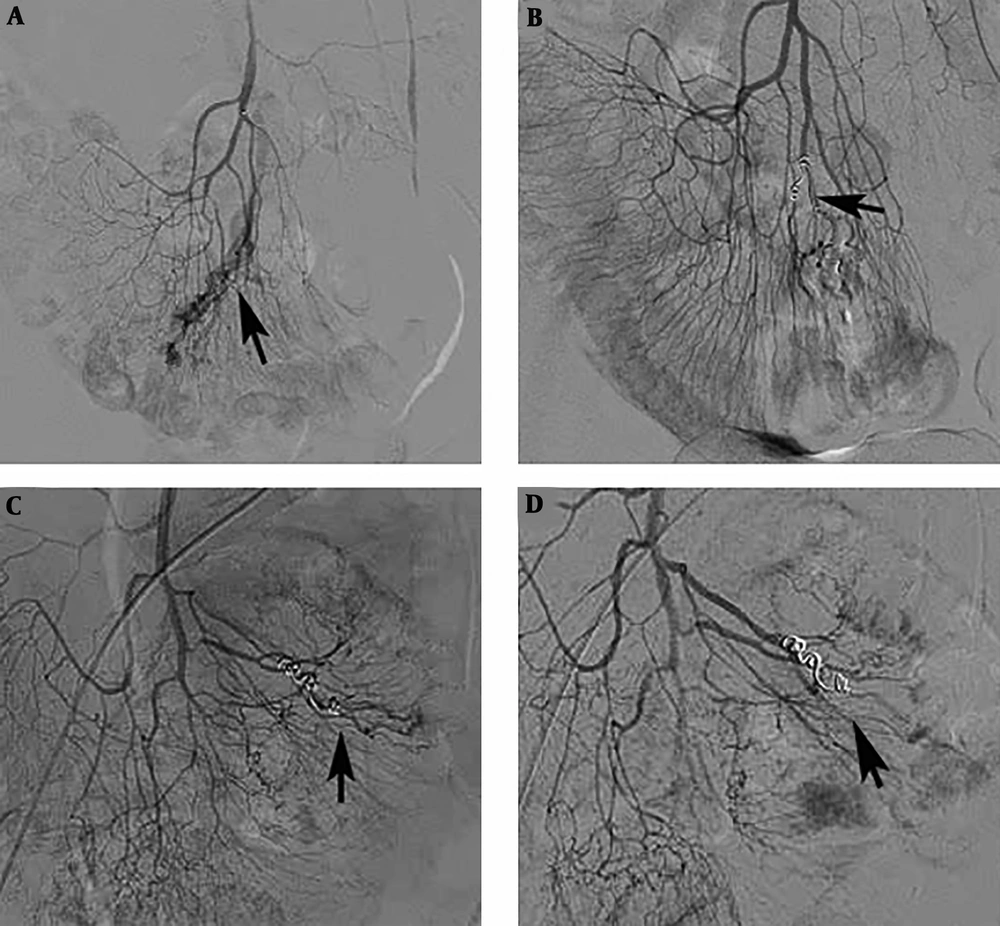
One patient with distal ileal AVM was diagnosed as hepatocellular carcinoma 10 months after the embolization, and underwent transcatheter arterial chemoembolization for tumor treatment. Angiography of the ileum was also performed, and no bleeding sites were detected (Figure 2).
A 75-year-old patient with an ileal angiodysplasia (AD). A, After superselective catheterization of the ileal artery was performed, angiography showed abnormal vascular tufts and an early venous phase (arrow); B, Microcoils were placed in the abnormal inflow artery of the ileal AD after the injection of polyvinyl alcohol (PVA) particles (arrow); C, Ten months later, repeated angiography displayed that there was still some remnant of the ileal AD (arrow); D, After injecting PVA particles (350 - 560 µm) into the abnormal vessels, the lesions almost disappeared (arrow).
5. Discussion
Following clinical assessment, endoscopy is often the first tool for diagnosis and treatment of acute GI hemorrhage (6). However, it is difficult to diagnose GI hemorrhage using endoscopy in the absence of active bleeding, particularly at the site of the small bowel, which is not easily accessible. In cases of severe bleeding, localizing the bleeding site by endoscopy is hard and the effect of endoscopic treatment depends heavily on the experience of the endoscopist. The failure rate of endoscopic diagnosis or treatment can be as high as 32% (7). Iron supplements, octreotide, and thalidomide are the main clinical medicine. However, it was reported that the effect of thalidomide alone or combined with oestrogen and progestin in treating hemorrhagic AD was not satisfactory (8). Enhanced CT is a useful modality for the diagnosis of GI hemorrhage, but it may fail when the bleeding rate is relatively low (9).
TAE plays an important part in diagnosing and treating GI bleeding. It allows accurate localization and immediately selective embolization for treatment, which may be more significant for patients with hemodynamic instability. The successful localization of the bleeding source by angiography depends heavily on the rate of bleeding. Generally, to enable diagnosis and treatment of GI hemorrhage, the bleeding rate is supposed to be over 0.5 mL/min during the examination (10).
The angiographic findings of AD may include tortuous vascular tufts and broadening or narrowing of blood vessels, an early venous phase, and signs of extravasation of contrast medium. But these are not features and are nonspecific in most cases. It is necessary to exclude diseases such as inflammatory bowel disease (IBD) and diverticular disease. It was reported that signs of increased staining of bowel loops and skip lesions are highly suggestive of IBD (11). A persistent vitellointestinal artery can be observed in patients with chronic GI hemorrhage due to Meckel’s diverticula (12).
In patients with repeated melena and chronic anemia, small AD lesions may be missed on angiography due to the vasoconstriction or vasospasm of the vessels responsible for the bleeding. In that case, superselective catheterization of the suspected vessels is required for diagnosis and treatment. Furthermore, repeated angiography can be performed to identify the lesions within several days, if necessary.
Transcatheter arterial embolization is an important tool for treating active GI bleeding, particularly when endoscopic therapy has failed and the patient is not eligible for surgery (13). Immediately following determination of the bleeding source by angiography, intraarterial embolization can be performed to stop bleeding by embolizing the abnormal vessels with materials such as coils, PVA particles, ethylene vinyl alcohol copolymer (onyx) glue, n-butyl cyanoacrylate (NBCA) and gelatin sponge particles (14-16). Although the occurrence rate of rebleeding is approximately 20% in patients with lower GI bleeding, immediate hemostasis can be successfully achieved by embolization at a rate as high as 96% (17). However, the risk of acute or chronic ischemia of the bowel due to excess embolization should be carefully considered. For this reason, microcatheters were chosen to superselect the specific anomalous vessels for the embolization procedure. As AVM and DL have higher bleeding rates than AE and the supplying arteries are relatively large, more kinds of embolic materials can be used in the embolization of AVM and DL than AE. In cases in which arteriovenous fistulas of AVM are very large, PVA or gelatin sponge particles should be avoided. The value of embolization is more notable for AVM and DL, but less for AE (1). The lesions of AVM and DL are usually more extensive than angioectasia. Endoscopic treatment is not suitable for the treatment of AVM due to the existence of arteriovenous fistulas, but it is more often used in the management of AE. In cases of DL and AE that endoscopic treatment fails to achieve hemostasis, angiographic embolization can be a preferential option.
To our knowledge, there are no guidelines for choosing embolic material to treat GI bleeding, and the selection is up to the operator. Pushable microcoil is the most widely used embolic material. In the present study, PVA particles (350 - 560 µm or 150 - 350 µm) were used as the dominant embolic agent. PVA particles are considered as a type of permanent embolic material, relative to gelatin sponge particles. The smaller PVA particles are more likely to reach the terminal branches of the vessels responsible for bleeding. PVA should not be used in cases that there are large arteriovenous fistulas or the artery anastomosis network is not good. However, the risk of intestinal ischemia increases if reflux into non-target vessels occurs. If the responsible arteries are relatively large and the bleeding rate is high, or in cases of rebleeding, PVA particles alone are not enough, and other embolic materials such as coils should be combined to reinforce the embolization. Onyx and glue may be a promising liquid embolic agent; however, controlling the glue penetration in vessels may be difficult and great experience with this technique may be required (15, 16).
Enterotomy is another method to manage AD. Nevertheless, most of the patients cannot accept surgery for reasons of large operative trauma, or are not eligible for surgery due to relative poor tolerance. If the bleeding cannot be controlled by endoscopy and TAE, surgery is supposed as the final therapeutic option. However, it may be hard to detect all lesions and to determine the true bleeding source during surgery. To achieve successful surgical resection, the preoperative or intraoperative localization of the lesions is required. TAE can serve as a useful tool for localization of GI bleeding origin by preoperatively implanting coils in the target vessels. The coil is not only palpable but also detectable under fluoroscopy during open surgery (14). Embolized coils can be retained in the responsible vessels for both treatment and localization purposes, which helps obtain additional time for determining optimal preoperative management for patients.
The present study has several limitations. First, it was a retrospective study. Second, the number of patients was relatively small due to rarity of the disease. Third, histologic proof of the diagnosis was unavailable. Moreover, the variety of clinical conditions of patients, lesions, embolic material, and techniques may be other limitations of the study.
In conclusion, for patients with refractory and repeated gastrointestinal hemorrhage due to angiodysplasia, TAE seems to be an effective alternative option when endoscopic examination and treatment do not work.