1. Background
Chronic kidney disease (CKD), especially that induced by diabetes mellitus (DM), and hemodialysis (HD) are commonly associated with vascular calcification (1, 2). The vascular calcification is classified into two types, medial calcification and intimal calcification, according to their histological and morphological characteristics. Medial calcification (Moenckeberg calcification) is induced by active osteochondrogenic formation in the middle layer of the muscular arteries and is particularly associated with diabetes DM, CKD and HD (3, 4). Diffuse medial calcification leads to arterial stiffness and resultant systemic hypertension (HT) (3). Intimal calcification is an aging plaque, shows eccentric location, and induces flow-limiting arterial stenosis (4). Both types of vascular calcification are related to peripheral artery disease (PAD) in patients with HD.
Several diagnostic imaging modalities are used to evaluate PAD associated with HD and to plan interventional procedures for PAD. Ultrasonography is a less expensive, easily accessible and noninvasive method for detecting vascular calcification (5). However, it is not feasible to demonstrate the vascular trees of the pelvis and legs comprehensively. Intravascular ultrasonography shows the distribution of medial calcification, other types of vessel plaques and luminal stenosis in patients with PAD (6). Its disadvantages are the invasiveness and limited coverage. Non-contrast-enhanced three-dimensional (3D) magnetic resonance angiography is useful for identifying leg artery stenosis without need of invasive procedures, contrast agents and irradiation (7). The drawbacks of this imaging method are its insensitivity to vascular calcification and lower spatial resolution.
Three-dimensional (3D) computed tomography angiography (CTA) is a powerful imaging tool for detection of vascular calcification and arterial stenosis with high spatial resolution in patients with PAD (8, 9). Maximum intensity projection (MIP) with and without subtraction and reconstructed transverse images allow for detailed assessment of the distribution of vascular calcification and luminal stenosis (10). Horimatsu et al. (6) evaluated the distribution of calcified plaque and plaque rupture in 159 patients with PAD using intravascular ultrasonography. They have demonstrated that calcified plaque can be less developed in the external iliac artery (EIA). So far, the distribution of medial calcification and arterial stenosis has not been evaluated by 3D CTA in patients with HD and PAD. The 3D CTA may give accurate information about the arterial disorders that could be treated by interventional procedures.
2. Objectives
The aim of this study was to determine the distribution of medial calcification and luminal stenosis in patients with HD and PAD using 3D CTA.
3. Patients and Methods
3.1. Patients
We retrospectively included 47 consecutive patients with HD and clinical symptoms related to PAD between January 2017 and December 2018. We included the HD patients presenting with intermittent claudication, leg coldness, phlegmon or gangrene of the foot or toes. Exclusion criteria were contraindication to iodine contrast agents and a history of bypass surgery, stent placement or amputation of the leg arteries before CTA. Informed consent was given by all patients, and our institutional review board approved this study.
3.2. 3D CTA and Postprocessing
The 3D CTA was performed using a 320-row multidetector CT (MDCT) system (Aquilion ONE Vision, Canon Medical Systems, Tochigi, Japan) or a 64-row MDCT system (Aquilion CX, Canon Medical Systems, Tochigi, Japan). For the 320-row system, an 80-row helical scanning mode was used with 0.5 mm slice thickness, pitch factor of 0.813, and gantry rotation of 0.75 s/rotation, while the 64-row helical scanning mode was used with 0.5 mm slice thickness, pitch factor of 0.828, and gantry rotation of 0.75 s/rotation for the 64-row system. The tube peal voltage was 100 kV and an automated exposure control was used, which led to approximately 100 - 600 mAs according to the tissue thickness. Precontrast CT was performed first, and subsequently 100 mL of iodine contrast agent with 300 - 370 mgI/mL was injected at a flow rate of 3 - 4 mL/s, followed by a 20 mL saline flash. The postcontrast scan timing was determined using bolus-tracking software (RealPrep, Canon Medical Systems) at the popliteal artery, which aimed to minimize the difference in luminal enhancement between the left and right legs and the contamination of venous return. When the arterial attenuation reached 150 - 200 HU or the artery was enhanced visually, the scan was triggered. HD was performed routinely after the 3D CTA.
Postprocessing methods used in this study were 3D MIP with and without subtraction and reconstructed two-dimensional (2D) transverse images. The 3D images without subtraction showed both the distribution of calcification and vessels, while that with subtraction showed patent arteries clearly. The subtraction images were acquired just by subtracting precontrast data from postcontrast data without any attenuation weighting methods followed by generation of 3D MIP. Reconstructed 2D transverse images showed the extent of calcification on the vessel wall. All images were generated by a team of radiological technologists on site.
3.3. Data Collection
The Radiology reports were recorded on site. Normal arteries were defined as patent arteries without calcification. Medial calcification was defined as circular calcification on the 3D MIP images without subtraction and 2D transverse images, which was not associated with significant luminal stenosis on the 3D images with subtraction (11). Luminal stenosis was defined as the decrease in the arterial diameter ≥ 70% on the 3D images with subtraction. The arterial system was divided to the common iliac artery (CIA), EIA, internal iliac artery (IIA), superficial femoral artery (SFA), anterior tibial artery (ATA) and posterior tibioperoneal arteries (TPA).
3.4. Statistical Analysis
The distribution of normal vessels, medial calcification, and arterial stenosis between the arteries was evaluated. In addition, we compared the distribution between the EIA and IIA or between the ATA and TPA, which branched off at the same level. A χ2 test was used for these analyses, and P value < 0.05 was defined as significant. When the numbers of normal arteries, arteries with medial calcification or luminal stenosis were beyond 75% (i.e., > 35) or below 25% (i.e., < 12) of the whole case series, the corresponding arteries were noted.
4. Results
The patient group consisted of 38 men and nine women with the mean age of 69.1 years (standard deviation [SD], 8.9 years; range, 51 - 87 years). The HD duration ranged from 1 to 42 years with a median of 7 years. Eighteen (38.3%) of the 47 patients had HT and 17 (36.2%) had diabetes mellitus (DM), and eight had both. Five patients (10.6%) had a history of chronic glomerulonephritis. Twenty-nine patients (61.7%) had a history of ischemic cardiac diseases, invasive treatment for the diseases or aortic valve replacement for calcified aortic stenosis.
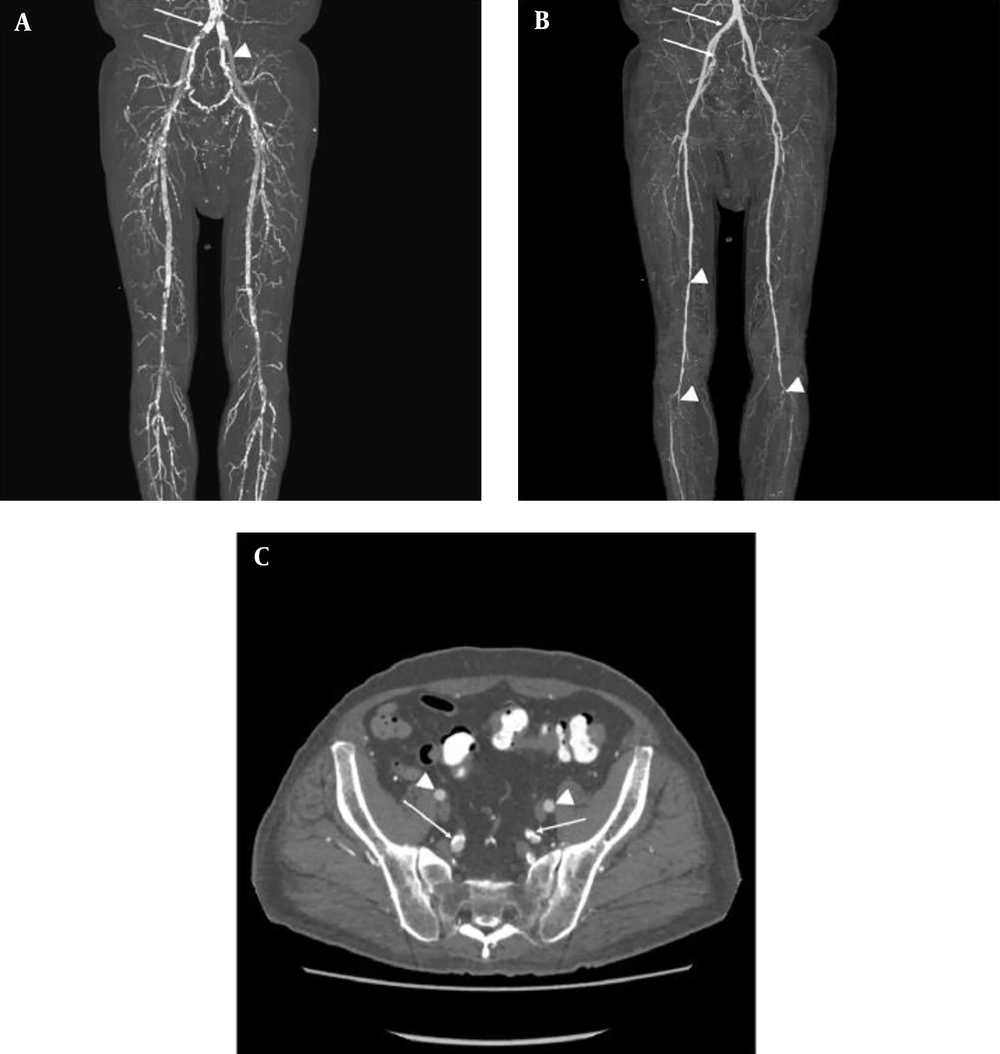
3D CTA examination was performed successfully and the postprocessed images were interpreted in all the 47 patients with HD and PAD. Table 1 summarizes the distribution of normal arteries, medial calcification, and arterial stenosis as seen by 3D CTA. Figure 1 shows a typical case of HD and symptomatic PAD. The distribution was significantly different between the arteries (P < 0.01). The EIA was spared from the both medial calcification and luminal stenosis (Figure 1). The distribution was also significantly different between the EIA and IIA (P < 0.01). There were no significant differences in the distribution between ATA and TPA (P = 0.71). The left EIA was normal predominantly even in patients with HD and symptomatic PAD. Conversely, the IIA, SFA, and TPA were normal only in a few cases.
Abbreviations: ATA, anterior tibial artery; CIA, common iliac artery; EIA, external iliac artery; IIA, internal iliac artery; l, left; r, right; SFA, superficial femoral artery; TPA, posterior tibioperoneal arteries.
aAll figures represent the numbers of cases presenting normal artery, medial calcification, or luminal stenosis of ≥ 70%.
bThe EIA was spared from vascular disorders, and the distribution was also significantly different between the EIA and IIA (P < 0.01, χ2 test).
cThe numbers of arteries were more than 75% of the whole cases (i.e., > 35).
dThe numbers of arteries were below 25% of the whole cases (i.e., < 12). The distribution was significantly different between the arteries (P < 0.01, χ2 test).
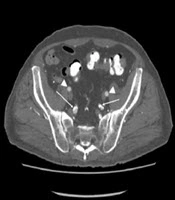
A 55-year-old man with left toe gangrene and history of hypertension, diabetes mellitus, and chronic myocardial infarction. He has received a 1-year hemodialysis. A, 3D maximum intensity projection (MIP) images show severe vascular calcification (arrows) and the preserved external iliac artery (EIA, arrowhead); B, 3D MIP images with subtraction reveals the patent common and internal iliac arteries (IIA), indicating the medial calcification of these arteries (arrows). By contrast, superficial femoral and tibioperoneal arteries are stenotic (arrowheads); C, Reconstructed transverse images show the preserved EIA (arrowheads). The IIA and its branches have medial calcification showing circular calcification without concomitant luminal stenosis (arrows).
5. Discussion
This study demonstrated that 3D CTA showed characteristic distribution of medial calcification and luminal stenosis in patients with HD and symptomatic PAD: the EIA was spared from both medial calcification and luminal stenosis and the prevalence of normal artery in the IIA, SFA and TPA was quite low.
The EIA was spared from both medial calcification and arterial stenosis even in symptomatic patients with HD and PAD. Horimatsu et al. (6) and Graziani et al. (12) have indicated that the EIA has vascular calcification or stenosis less frequently in patients with PAD or HD. We speculate that relatively high blood flow, straight pathway, and fewer branches (i.e., inferior epigastric and deep circumferential arteries) of the EIA contribute to its resistance to vascular calcification. By contrast, the IIA with tortuosity and many branching had either medial calcification or luminal stenosis. The IIA, SFA, and TPA with many muscular branches and slower blood flow showed arterial disorders frequently. A previous study indicates that these arteries tend to be stenotic even in the unselected elderly subjects (13). In another study, SFA is treated by interventional procedures much more frequently than the CIA and EIA in patients on chronic HD (12). Our results were consistent with these previous studies. In some previous studies, the presence of vascular calcification is found to affect the outcomes of patients with HD or PAD despite the development of interventional techniques and metallic stents (11, 12, 14). Identifying arterial disorders by 3D CTA may be valuable for planning the revascularization therapies and evaluating arterial stiffness and its complications such as HT, myocardial hypertrophy, and myocardial ischemia on exercise (1, 6, 14, 15).
This study had limitations. First, we did not include HD patients without symptoms related to PAD or who had undergone revascularization therapies or amputation before CTA. Thus, the exact frequency of PAD or medial calcification associated with HD remains unknown. However, the use of CTA and iodine contrast agents may not be warranted in HD patients without clinical symptoms, for whom ankle brachial index or ultrasonography should be assessed when screening for PAD. Second, we analyzed Radiology reports made on site, but did not interpret 3D CTA images in a blinded manner. The present study was also descriptive and retrospective, which might lead to selection bias. Nonetheless, the present study may reflect the clinical scenario of these patients. Third, a histologic confirmation of medial or intimal calcification was not made. Medial calcification was defined according to a previous study (11). Finally, because of the retrospective, cross-sectional study design from a single center, the effects of medial calcification or luminal stenosis on the HD patients’ prognosis could not be determined.
In conclusion, the distribution of medial calcification and luminal stenosis characteristic of PAD associated with HD are visualized using 3D CTA. The EIA is spared from medial calcification and luminal stenosis. The IIA, SFA, and TPA tend to show either medial calcification or luminal stenosis. The characteristic distribution may be related to the vascular diameter, pathway, number of branch arteries, and blood flow. Identification of arterial diseases by 3D CTA may assist the planning of revascularization therapies in symptomatic patients with HD and PAD.