1. Background
The important cause of late discharge from the hospital after surgery is pain. The experience of pain is complicated and multifactorial, and it is a repulsive feeling and emotional experience. In addition, pain is a personal and subjective experience that involves sensory, psychological, and behavioral factors linked to tissue damage. Behavioral differences in phenotype, age, background of culture, genetics, and type of surgery, and physiological factors such as fear, anxiety, and depression can be effective in responding to surgical injuries (1).
After the surgery, more than 70% of patients suffer moderate to severe pain, and more than 25% of patients feel complications after taking painkillers (2). Postoperative pain control is a significant concern for physicians and patients experiencing surgery (3). Ineffectual and adverse postoperative pain control raises the risk of chronic pain (3, 4). Also, it can provoke sleep disturbances, decrease respiratory movements, handle coughing and sputum secretion (5). It can also induce infarct, myocardial ischemia, pulmonary infection, ileus, urinary retention, thromboembolism, weakened immune function, and anxiety. Besides, it secondarily provokes patient dissatisfaction and harm to patients' faith and long-term hospitalization and increases care expenses (3). Pain control is particularly critical in orthopedic patients because the adverse pain control in them can be followed by slowed mobility and joint movement (2-4). Various procedures may be practiced to control pain during surgery for orthopedic surgery patients (5).
There are some mechanisms associated with postoperative pain, based on each, different classes of judges are presented for treatment or prevention. Postoperative pain can induce hypersensitivity and undue pain perception and can turn acute postoperative pain into chronic pain (5). The most significant drugs employed to control pain are narcotics, which have some limitations due to their moderately high and sometimes intolerable side effects such as itching, nausea, and complications of respiratory failure. The painkillers used are a wide variety of drugs such as opioids, cyclooxygenase 2 inhibitors, gabapentin, pregabalin, alpha-2 agonists, and anticonvulsants. A combination of drugs with different multimodal techniques is preferred to single-drug therapy (5, 6). Intravenous ketorolac drugs are used alone or in combination with opioids to control postoperative pain successfully (7-10). Ketorolac is a non-steroidal anti-inflammatory drug with analgesic features. This medication represses both the enzyme lipoxygenase and cyclooxygenase. It is also available in both edible and injectable forms. Prophylaxis with preoperative analgesics can decrease the need for analgesia through surgery and lessen postoperative pain (7, 8, 11, 12). Dexamethasone, on the other hand, is a strong anti-inflammatory drug with thirty to forty times the power of hydrocortisone and up to sixteen times the potency of prednisolone (13). Given that there has been no study comparing the effect of dexamethasone and ketorolac on pain control in elective foot surgery, the present study seeks to answer the question of "between dexamethasone and ketorolac, which one is more effective in controlling pain after ankle surgery?"
2. Methods
This study was a double-blind, randomized clinical trial. After the permission of the Medical Ethics Committee of Shahid Beheshti University of Medical Sciences, this study was performed for six months in Akhtar and Imam Hossein Hospitals with the available sampling method in 1398. Out of a total of 53 patients who were referred to Akhtar and Imam hospitals with ASA class one and two in the age group of 20 to 60 years, 40 candidates were selected and were divided into two groups.
Criteria for entering the study: (1) being in the age group of 20 to 60 years; and (2) a candidate for orthopedic surgery of the lower limbs. Criteria for withdrawal: (1) pregnant and lactating women with impaired renal function or dialysis; (2) heart disease; (3) use of warfarin during treatment; (4) contraindications to opioid medications, hemorrhage, especially gastrointestinal hemorrhage; (5) history of high blood pressure; (6) history of anemia and thrombocytopenia; (7) drug addiction; and (8) asthma and lung disease and allergies to NSAID. The ethical criteria of this study were obeyed by acquiring a license from the Ethics Committee of Shahid Beheshti University of Medical Sciences.
Throughout the research phase, the purposes of the study were explained to patients. It was also stated that they can be excluded from the study whenever they want, and their lack of collaboration with the treating physician and the hospital would not influence their treatment, and all patient’s data would be retained confidential. All patients filled and signed an informed consent form to participate in the study. First, all patients received 10 mg of oxazepam at 6 a.m. as a pre-medication. After being carried to the operating room, the patients were monitored, two intravenous pathways were implanted, and they were fluid-therapied with 5 ccs/kg Ringer's lactate. Anesthesia induction was then started with general anesthesia for patients by injecting fentanyll 1.5 mg/kg, sodium thiopental 5 mg/kg, and atracurium 0.5 mg/kg. After intra-chip intubation, the anesthesia was maintained with a perforated infusion of 2 μg/kg/min, and patients were mechanically ventilated throughout the surgery. Upon entering the operating room, blood pressure, heart rate, and SPO2 were monitored in all patients. The items were re-monitored after induction, throughout the operation, at the end of the surgery, and after the patients entered the recovery room. After the surgery, the patients were randomly split into two groups of 20 based on a table of random numbers. Patients in the first group received 8 mg of dexamethasone intravenously. Also, for the second group, 90 mg of ketorolac (Caspian Tamin Rasht Company) was infused in 1 liter of normal saline serum within 24 hours. Painkillers were injected into patients for recovery by an anesthesiologist. While the patients were not aware of the type of medicine they were receiving (first type blindness). During 24 hours of patient monitoring, postoperative pain evaluation was initially performed at recovery time in 0, 2, 4, and 6 hours after the injection by an unaware intern and by VAS. For 6 hours, patients were monitored for pain intensity and the need for meperidine injection. If patients had a pain score higher than VAS 4, they received 25 mg of muscle protein. Patient nausea and vomiting were also evaluated for 12 hours after surgery, and a qualitative score of 1 - 4 was given.
2.1. Visual Analog Scale (VAS)
This scale indicates the pain of patients generally. This measure is drawn as a 10 cm line, and the amount of pain is depicted between 0 and 10 cm. 0 shows no pain, 1 to 3 is mild pain, 4 to 6 moderate pain, and 7 to 10 severe pain (14). The reliability of this tool is told to be 85 to 95% (15). In data analysis, mean, standard deviation, frequency, table, and graph were employed to sort and summarize the gathered data. In the study of statistical assumptions, according to the number of observations in each distribution, to test the naturalness of data distribution, the Kolmogorov-Smirnov test was used. Given the satisfaction of statistical expectations, the independent t-test was used at a 95% confidence level, and the statistical package version 22 was used.
3. Results
Out of 53 patients who were candidates for elective foot surgery, 40 were picked based on the entry criteria. The two groups have no important difference in terms of demographic characteristics. The patients’ characteristics are presented in Table 1.
| Variables | Dexmedetomidine (n = 25) | Control (n = 20) | P-value |
|---|---|---|---|
| Age (y) | 49.3 ± 10.4 | 51.4 ± 11.2 | 0.68 |
| Gender (%) | 0.27 | ||
| Men | 11 (27.5) | 10 (25) | |
| Women | 9 (22.5) | 10 (25) |
The results of the Kolmogorov-Smirnov test revealed that the data distribution was normal (P > 0.05). The independent t-test was used to match pain levels in the two groups of ketorolac and dexamethasone. Evaluation of nausea and vomiting in 0 to 6 hours showed that the incidence of this complication in ketorolac infusion group was significantly lower than intravenous dexamethasone group so that three patients in the ketorolac group and 12 patients in the dexamethasone group had nausea, and vomiting was monitored in all patients (P < 0.05).
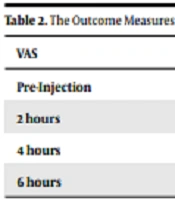
The results of the t-test proved no important difference between the two groups in terms of VAS before the intervention (t = 0.354, P > 0.05, Table 2). But in two and four hours after the injection, there was a significant difference between the two groups in terms of VAS (P < 0.05, Table 2): the pain was weaker in the ketorolac group than in the dexamethasone group. However, six hours after the injection, the pain difference was not statistically significant between the two groups.
| VAS | Ketorolac (n = 20) | Dexamethasone (n = 25) | P-Value |
|---|---|---|---|
| Pre-Injection | 8.55 ± 0.51 | 8.6 ± 0.5 | 0.56 |
| 2 hours | 2.6 ± 0.74 | 4.8 ± 0.68 | 0.038 |
| 4 hours | 1.8 ± 0.74 | 4.5 ± 0.8 | 0.04 |
| 6 hours | 2.8 ± 0.88 | 5.3 ± 0.74 | 0.89 |
z Abbreviation: VAS, visual analogue scale.
4. Discussion
The purpose of this study is to consider the effect of dexamethasone and ketorolac on pain control of elective foot surgery. The findings revealed that in two and four hours after the injection, there was an important difference between the two groups in VAS: the pain was less in the ketorolac group than in the dexamethasone group. However, six hours after the injection, the pain difference was not statistically significant between the two groups. Ketorolac, a potent and regularly used nonsteroidal anti-inflammatory drug (NSAID) in orthopedic operation, has been shown to be a valuable non-narcotic drug for controlling postoperative pain (16-18).
Ketorolac is a non-steroidal anti-inflammatory drug, derived from arachidonic acid that interferes with the synthesis of inflammatory and pain mediators. Moreover, NSAID analgesia is commonly attributed to peripheral suppression of cyclooxygenase (COX) enzymes, which causes decreased activity of the arachidonic acid cascade, with possible additional mechanisms of action. The local accumulation of prostaglandins (PGE) E and I2 is a direct result of surgical trauma and leads to the sensitization of the nociceptors of the A-TM and C fibers. Inhibition of prostaglandin synthesis at the region of injury decreases sensitization and leads to a reduction in postoperative pain. PGE2 is made of both COX-1 and COX-2, and ketorolac effectively suppresses both enzymes. NSAIDs might produce analgesic effects with mechanisms independent of suppression of peripheral prostanoid levels. Glucocorticoids also suppress COX-2 by transcriptional and post-transcriptional mechanisms (19).
Ketorolac was used as a potential preemptive analgesic agent. One feature of Ketorolac is its reasonable efficacy (20); the use of intravenous or intramuscular is a simple route for administration; with even a single dose of opioid may happen acute tolerance and severe cardiorespiratory or central nervous system complications (21). In patients with risk factors for renal dysfunction and procedures with minimal blood loss (such as ankle fracture repairs using a tourniquet) prescription of ketorolac should offer a very suitable margin of safety. Previously, ketorolac has been shown to improve early tourniquet tolerance and quality of postoperative anesthesia when used in combination with lidocaine for IVRA. The first report of IVRA with ketorolac was in 1992 by McQuay et al. (22). A study by Reuben et al. in 1995 reported significant perioperative benefits from IVRA-ketorolac compared to systemic controls. They found that considerably fewer patients had a pain score of > 3/10 during the first 30 and 60 min after surgery when 60 mg ketorolac was added to lidocaine IVRA, compared to a group who received 60 mg ketorolac and lidocaine IVRA (23). The study by Vanos et al. (24) found that ketorolac was similarly effective either as an infiltrate into the surgical site before incision or when given as an adjunct to IVRA. Surgical site hematomas, which are the major undesirable side effects attributed to ketorolac as a part of IVRA, were not observed in this study.
Reuben et al. (23) and Rogers et al. (25) have previously examined the potential preemptive analgesic effects of ketorolac. Fletcher et al. (26) and Kissin (27) showed that the use of 10 mg ketorolac intravenously before skin incision decreases opioid usage after surgery. In general, according to the results of the research, it can be concluded that ketorolac is a better drug in foot surgery than dexamethasone to control pain.