1. Introduction
Erector spinae plane block (ESPB) is a regional anesthetic technique involving local anesthetic (LA) injection in a paraspinal plane deep into the erector spinae muscle spreading the drug to multiple paravertebral spaces (1). The ESPB was initially described in 2016 for thoracic neuropathic pain when performed at the T5 level. More recently, ESPB has also been shown to be effective in providing extensive somatic and visceral abdominal analgesia when performed at the T7-9 level (2).
The great popularity of ESPB guaranteed by its simple execution and its safety profile has allowed expanding the range of applications, including the treatment of acute and chronic pain. Although the exact mechanism of ESPB requires further study, some postulate that it targets the ventral branches of spinal nerves providing both somatic and visceral blockades (3).
Recent magnetic resonance imaging studies have demonstrated that visceral and somatic analgesic effects provided by ESPB result from the transforaminal and epidural spread. This finding explains the visceral pathway and the multiple spinal segmental blockades through the circumferential epidural spread (4).
Epidural analgesia is considered the gold standard for postoperative analgesia in major open abdominal surgeries. Nevertheless, using ESPB avoids epidural analgesia side effects (e.g., hypotension, motor block, and risk of major complications) and lowers the opioid consumption, decreasing the rate of side effects. Bilateral continuous ESPB might prolong analgesia by LA infusion through a catheter positioned in the target plane, representing a valid alternative to epidural analgesia.
Multiple indications of ESPB are linked to its spread to the paravertebral region, taking advantage of the distribution of the erector spinae muscle from the neck to the lumbar region. Lumbar ESPB has been recently introduced as an alternative to postoperative analgesia. In this case report, we describe ultrasound-guided lumbar ESPB at L2 for intraoperative and postoperative analgesia in a middle-aged female case scheduled for open surgical repair of iatrogenic ureteral bilateral injury after hysterectomy.
2. Case Presentation
We describe the case of a 46-year-old female patient (weight: 57 kg, height: 164 cm, body mass index (BMI): 21.2 kg/m²) who underwent bilateral open ureteral reimplantation surgery for iatrogenic ureteral injury at the Civil Hospital of Teramo in Italy (January 2022).
History of thyroidectomy and radioactive iodine treatment for papillary cancer, melanoma, hysterectomy, and left adnexectomy 4 months ago was reported. Following the gynecological surgery, she presented anuria. Abdominal computed tomography angiography showed retroperitoneal spillage of iodinated urine bilaterally with visualization of both ureters only up to the lumbar region. After unsuccessful attempts of bilateral ureteral stenting and bilateral nephrostomy placement, bilateral ureteral reimplantation was indicated.
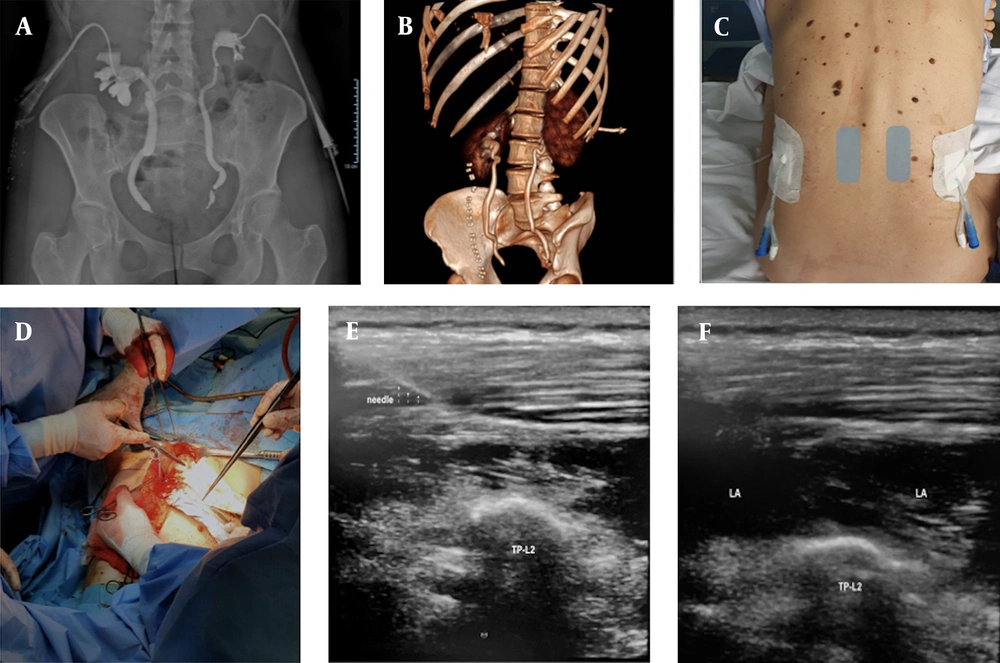
Preoperatively, after premedication with midazolam 2 mg and fentanyl 0.05 mg, ultrasound-guided bilateral lumbar ESPB (L2) was performed to gain adequate intraoperative and postoperative pain control. With the patient in a sitting position (Figure 1C), a high-frequency linear probe in longitudinal orientation (13 - 6 MHz, SonoSite, Amsterdam, Nederland) was placed at about 3 cm from the midline to identify the deep plane of the erector spinae muscle. Subsequently, a 50 mm 22 G echoreflective needle was inserted with an in-plane approach and craniocaudal direction. After hydrolocation by 3 mL of saline, the anesthetic solution (levobupivacaine 0.375% plus 30 mcg of clonidine in 20 mL saline solution per site) was injected into the target fascial plane (Figure 1E).
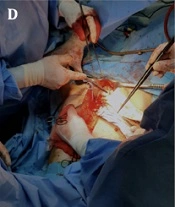
(A) preoperative pyelography; (B) postoperative three-dimensional computed tomography reconstruction; (C) patient sitting position, presence of bilateral nephrostomy, ultrasound probe positioned on grey rectangles; (D) surgical field; (E) TP-L2: transverse process of L2; (F) LA: local anesthesia; TP-L2: transverse process of L2.
The induction of general anesthesia was conducted with propofol (2 mg/kg), fentanyl (0.05 mg), and rocuronium (0.6 mg/kg). Standard intraoperative monitoring included the bispectral index (BIS) and train of four (TOF). For maintenance, balanced anesthesia with sevoflurane (minimum alveolar concentration = 0.8 - 1) and remifentanil infusion (0.06 γ/kg/minute) was chosen. Remifentanil infusion was interrupted intraoperatively 15 minutes after surgical incision for good surgical pain control under BIS monitoring (within the range of 38 - 45). Supraumbilical pubic incision, ureters identification and debridement from adhesions, 1.5 cm section of the left ureter, and placement of bilateral stents with subsequent ureters anchorage to the bladder were performed (anesthesiologic/total surgical time of 260 minutes).
A postoperative analgesic therapy with reduced opioid doses was chosen, and a preemptive bolus of paracetamol 1 g, ketorolac 30 mg, and morphine 5 mg was administered, in addition to antiemetics and gastroprotection drugs. An infusion pump with morphine 10 mg, ketorolac tromethamine 60 mg, and metoclopramide 10 mg (total volume: 50 mL, rate: 2 mL/hour) was also prepared but not administrated in the postoperative period.
On awakening, the patient maintained stable vital parameters. Moreover, good pain control (numerical rating scale [NRS] = 0), no postoperative nausea and vomiting, and no hypotension were recorded. Postoperative pain evaluation at 3, 6, 12, and 24 hours in static (at bed supine/sitting position) and dynamic (at side/standing mobilization) conditions reported a substantial absence of pain with NRS = 0 and NRS = 1 - 2 in the dynamic component. The patient was promptly mobilized, and the postoperative course was free of complications. No lower limb weakness or motor block and bladder spastic pain were reported in the postoperative period. Satisfaction and comfort with the anesthetic technique were also reported by the patient in the postoperative period.
3. Discussion
The great success of ESPB since its first description has led to a flourishing of new indications for analgesia/surgical anesthesia and as treatment of chronic and acute pain. The possibility of performing ESPB rapidly extended to the lumbar and cervical sacral areas is reported in several experiences (5). However, few studies and detailed reviews, including indications, efficacy, mechanism of action, and limitations regarding the lumbar ESPB, are present in the literature (6). The ESPB’s aim is to penetrate the anterior layer of the erector spinae muscle and deposit the injectate between this layer and the tip of the transverse process to allow a craniocaudal spread in multiple spinal segments. To the best of our knowledge, this has been the first described case of lumbar ESPB for intraoperative and postoperative analgesia in bilateral ureters replacement and reimplantation after iatrogenic injury in an adult.
Few experiences regarding the use of lumbar ESPB are described in pediatric urological surgery, with good postoperative pain control both with single-shot technique and continuous ESPB in open pyeloplasty/ureteral stent insertion and open prostatectomy. Postoperative pain related to ureter reimplantation can be a challenge, the pain can be severe due to the somatic component of the surgical wound and visceral component with spastic bladder pain. The caudal block compared to the high epidural seems to be more effective in spastic visceral pain (7).
The mechanism of postoperative bladder spasms genesis following surgical insult of strain and chemical irritants is not well understood. The complex somatic, visceral, and autonomic innervation (parasympathetic afferents from S2-S4 and sympathetic afferents from T11-L2 segments) of the bladder and lower abdomen/perineal area are involved in somatic and visceral spastic postoperative pain. Other locoregional techniques of fascial blocks are proposed for the lower abdomen, such as the transversus abdominis plane and quadratus lumborum block; however, no prospective study comparing the various techniques has been published.
The erector spinae muscle at the lumbar level is thicker than in the thoracic area, making lumbar ESPB under ultrasound technically challenging. Herein, we chose an in-plane parasagittal approach to perform ESPB with the patient in the sitting position due to the presence of bilateral nephrostomy (Figure 1C and E), which would have made a transverse out-of-plane or in-plane approach difficult in a lateral or prone position. Numerous reports described sitting position for homogeneous extensive cephalocaudal LA spread. A mixture of 0.375% levobupivacaine (20 mL per site plus 3 mL of hydrodissection) with the addition of clonidine (30 mcg per side) single-shot allowed to maintain deep anesthetic plan only with sevoflurane, stopping remifentanil infusion during the first half-hour of surgery. During postoperative management, the absence of pain allowed a real opioid-sparing, probably due to the long levobupivacaine duration of action (rarely described) and of clonidine as an adjuvant.
The absence of bladder spastic pain, throughout the postoperative period, without the appearance of muscle weakness in the lower limbs, made us exclude the extension of the block at the lumbar plexus, paravertebral, and epidural space but not the LA diffusion through near anatomically contiguous structures of quadratus lumborum and psoas fasciae. Pain control in the visceral spastic component made us hypothesize a wide LA spread in the craniocaudal sense that also involved the sacral and thoracic areas or even anterior/circumferential spread up to the sympathetic or parasympathetic chain, as reported in a nuclear magnetic resonance imaging study (8).
No symptoms attributable to local anesthetic systemic toxicity (LAST) were recorded. However, it was reported when performing ESPB with more than 40 mL of diluted LAs. High anesthetic mixture volumes and concentrations might be directly proportional to the ESPB success rate. It is necessary to obtain clinical data for optimal dose-volume regimens considering patient conditions, injection sites, and types of LA.
The wide variability and unpredictability of LA spread between the fasciae have been described and investigated both in the cadaver and in clinical/ radiological studies (9).
The LA spread is probably linked to the volume of anesthetic injected solution, the anatomical characteristics of the column districts, and the individual factors (9). This could explain the high variability of ESPB-sensitive block extension and even probably its limit. The ESPB and all interfascial plane blocks seem to be relatively safe from LAST due to the low vascularity of fascial structures (5). Instead, in the bilateral ESP block, it is possible that the LA spread will not be distributed equally between the two sides as reported, an event that did not occur in our case.
The use of bilateral lumbar ESPB for ureteral reimplantation (i.e., iatrogenic or posttraumatic) in adults could represent a valid indication for pain management. The lumbar ESPB target is the transverse process and is relatively distant from important nerve and vascular structures. Lumbar ESPB also offers advantages when conditions, such as coagulopathy or anticoagulant drugs, contraindicate paravertebral or epidural block. In the present case, the possibility of extending the duration of the single-shot analgesic block by adjuvants, such as clonidine, dexmedetomidine, or dexamethasone (10), represents a further advantage in terms of opioid-sparing in the perioperative period. Clonidine is a powerful alpha-2 agonist that prolongs block duration, allowing the reduction of opiates and its side effects (i.e., bradycardia, hypotension, nausea, vomiting, and itching), as in the present case.
The use of bilateral lumbar ESPB, as already reported for numerous lower abdominal surgeries, can be a valid alternative in the perioperative management of pain, especially in patients who can benefit from opioid-free anesthesia/analgesia. The prompt mobilization of the patient and the rapid canalization can represent an undisputed advantage in patients with respiratory problems or serious comorbidities.
The focus in the clinical practice is always patient safety. The safety and reliability of one technique over another cannot be established with a single case. It would be desirable to design studies that compare various regional and neuraxial anesthetic techniques to better establish and highlight efficacy, safety, and reliability in the various types of surgery. Having in armamentarium the techniques as lumbar ESPB described, which are easy to perform, effective, and relatively safe for patients, represents the goal of future research in this field.