1. Context
1.1. Bioinformatics
Utilizing computer technology to gather, store, analyze, and share biological data and information is referred to as bioinformatics, which is related to genetics and genomics (1, 2). Biological queries have been analyzed in silico utilizing computational and statistical methods and bioinformatics. The biological information must be merged to provide a complete view of these processes to examine how common cellular functions are changed in various disease states (2, 3). The analysis and interpretation of diverse sorts of data is presently the most urgent challenge in bioinformatics due to how the subject has developed. Bioinformatics' main purpose is to improve our understanding of biological interactions. However, what distinguishes it from alternative strategies is its emphasis on creating and utilizing computationally expensive procedures to attain.
1.2. Immunoinformatics
A significant amount of information related to immunological explorations has been produced as a result of the sequencing of genomes of human or alternative model organisms. In addition, a great deal of clinical and population-level information is being collected as different kinds of scientific evidence and clinical information. Such data aggregation is regarded as a valuable origin for scientists looking for processes of immunological function and illness immunopathogenesis. Hence, the necessity to deal with this quickly emerging immunological resource has laid the foundation for the field known as immunoinformatics. Immunoinformatics, also called computational immunology, denotes the interconnection of computational knowledge and experimental immunology. This field involves the use of computerized approaches for comprehending immune system data. In addition to aiding the management of large quantities of information, this field contributes to defining novel hypotheses linked to immune feedback. Immunoinformatics can accelerate the design of vaccines and immune-mediating therapeutics. The present review provides an up-to-date discussion and suggestions on the novel role of immunoinformatics in neurosurgical practice. In addition, we provide insights into further studies about the COVID-19 pandemic.
2. Evidence Acquisition
We searched PubMed and Google Scholar without time restrictions. The keywords used included “immunoinformatics,” “in silico,” “neurology,” and “neurosurgery.”
3. Results
3.1. Overview of the Application of Bioinformatics and Immunoinformatics to Neurosciences
Neuroinformatics contributes to creating and maintaining web-reachable databases of computational and experimental data, in line with unique software tools, which are crucial for comprehending the normal operation and neurological disorders of the nervous system. Neuroinformatics comprises traditional Bioinformatics of gene and protein sequences in the CNS; atlases of CNS anatomy as well as identification of protein and genetic sequences; neuroimaging by functional magnetic resonance imaging (fMRI) and electroencephalography (EEG), positron emission tomography (PET), and magnetoencephalography (MEG).
In addition to these conventional immunoinformatics techniques, computational immunology is an even more multidisciplinary tool for targeting the immune system to enhance neurosurgical practice. In the following section, we cover two key instances of targeting brain tumors using in silico vaccine research and genetics pathway analysis, as well as immunomodulation by immunoinformatics design of candidates to target biofilms (4).
AI could also aid in the processing of images of MRI, for example, for migraine detection and treatment (5).
3.2. Bioinformatics and Immunoinformatics in Neurosurgical Practice
Glioblastoma multiforme (GBM) is an invasive primary cancer in adults that is the most prevalent high-grade glioma of the central nervous system (CNS). Cytotoxic T lymphocytes (CTLs), Helper T lymphocytes (HTLs), and cytokines, including IL-12, IL-13, IFN-, IFN-, and IFN- are related to GBM (6). A common treatment is the complete excision of tumors. However, removing tumors may not be possible due to infiltration into other nearby tissues. After surgery, patients may undergo various therapies, such as chemotherapy, cytotoxic treatment, and radiotherapy. Recently, immunotherapy could be used for the therapy of GBM (7). However, its effects are inadequate and offer limited protection.
Vaccination, the primary immunotherapy strategy for GBM, increases the immune response of the body toward tumors by injecting foreign antigens. Protein immunogens are tumor-specific antigens, such as EGFRvIII or heat-shock proteins, injected intravenously. The delivery of ex vivo-engineered cells is required for cell-based vaccinations, most of which are dendritic cell (DC) and cancer cell vaccines. In contrast to the excellent outcomes of stage I/II clinical trials, hardly any stage III clinical trials targeting immunotherapies for glioblastoma have yet been approved.
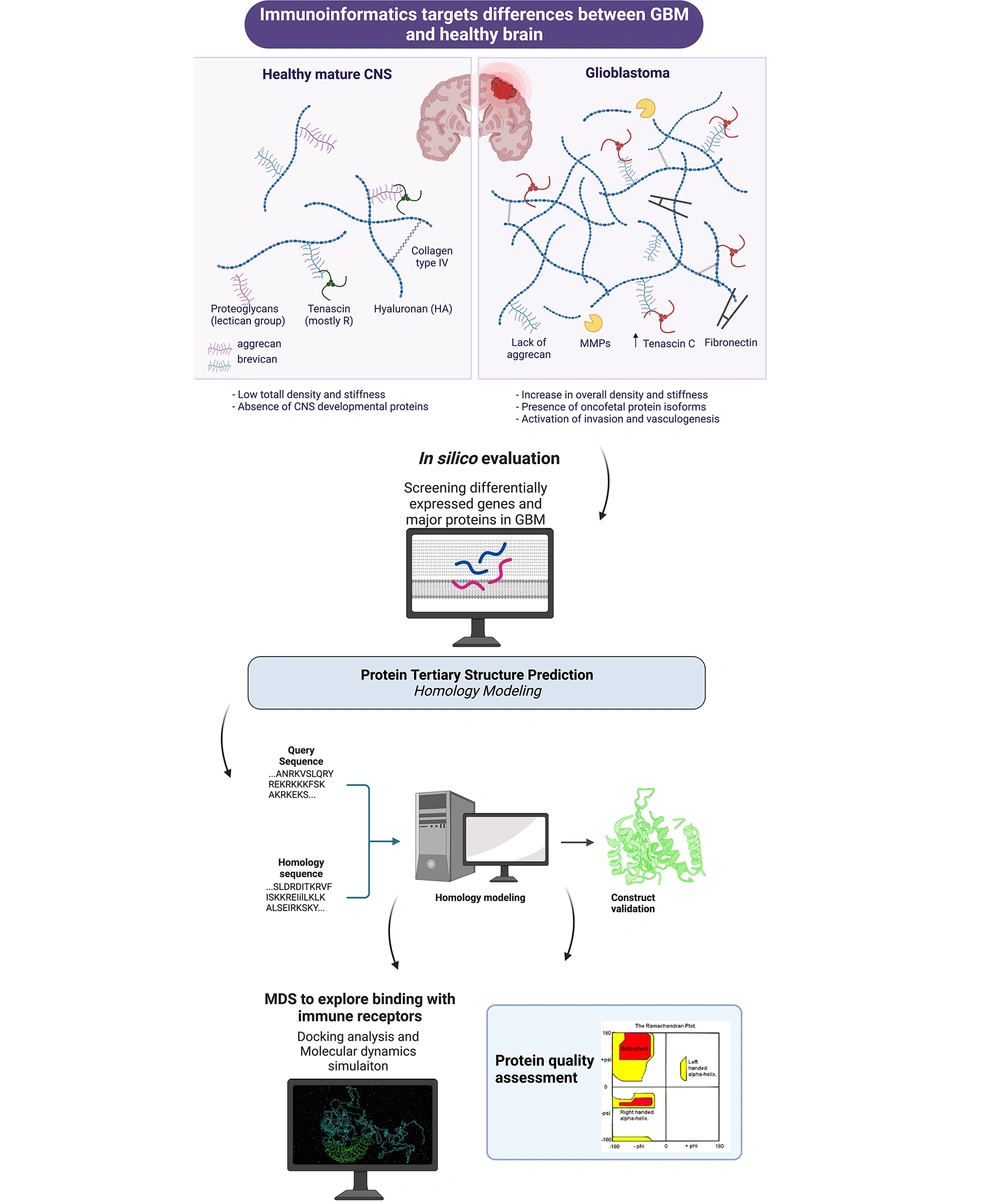
Immunotherapy is vastly important as a safe and effective strategy for preventative or therapeutic reasons against glioblastoma multiform (8). Bioinformatics can expedite vaccine development. Several phases are involved in the design of therapeutic protein vaccines, including retrieval of amino acid sequences, prediction of helper T-lymphocytes (HTL) and cytotoxic T-lymphocytes (CTL) epitopes, prediction of B-cell epitopes, evaluation of population, physicochemical characteristics, and homology modeling, molecular docking with immune receptors, and molecular docking simulation (MDS) (9-11). A brief overview of immunoinformatics application to GBM is provided in Figure 1 (Created with BioRender.com).
A new study aims to develop a vaccination against glioblastoma multiform that targets many regions. The most potent areas of proteins that stimulate B- and T-lymphocyte immune responses against glioblastoma multiform were identified using an in silico technique. IL-13R-2 (amino acid locations 27 - 144), PTPRZ-1 (amino acid positions 731 - 884), and TNC (amino acid positions 1900 - 2100) exhibited highly inducible immunological responses. The researchers used them for linking using a linker A(EAAAK)3A to create a multi-regional immunogenic candidate. The immunoinformatics evaluation of the constructed recombinant vaccine was conducted to determine its effectiveness. The antigenicity of the developed recombinant vaccine was around 0.78, despite the absence of allergen characteristics. Characterization of the physicochemical characteristics of the recombinant vaccine construct demonstrated the efficacy of the vaccine candidate. Immunoinformatics methods such as molecular docking, secondary and tertiary structure prediction, mRNA structure prediction, and immunological simulation were then performed for further evaluation. Following this, the intended recombinant vaccine structure was made, cloned into the pET28a vector, and generated in E. coli BL21. In addition, circular dichroism spectroscopy was employed to investigate secondary alterations in the structure of the recombinant vaccinate. Validation analysis of the expression of the recombinant vaccine structure showed that the Bioinformatics analysis was fairly accurate. In future studies, immunogenic candidates validated by immunoinformatics techniques should also be tested in preclinical settings (6).
Another method in silico analysis that helps GBM research is pathway enrichment analysis. A recent work performed in silico evaluation of alterations in the immune setting during malignant progression of IDH-mutant low-grade glioma. This experiment found that the dominant immunological subclass in low-grade glioma was M2 macrophages. Following these macrophages, triggered monocytes and mast cells were the most dominant. Also, CD4+ T-lymphocytes were higher than CD8+ T-lymphocytes in the tumor microenvironment (TME) of low-grade gliomas. When gliomas shifted towards malignancy, induced mast cells, natural killer (NK) cells, and monocytes were reduced. Immunity-associated genes were distinctly expressed in macrophages following malignant conversion. A major rise in progression-free survival was found in cases that showed an elevation in induced plasma cells or NK cells. This work shows case-unique alterations in immunological cell subgroups during the malignant alteration of IDH-mutant low-grade glioma (12).
The increasing prevalence of neurosurgery implants is being used in a rising subpopulation of senescent patients with numerous morbidities due to the fast development of neurosurgical practice. As a result, neurosurgical implant-related infections are rising, resulting in significant morbidity and mortality, including noticeable bone abnormalities and a lack of CNS protection. Biofilms, which are difficult to eradicate, are present in all implant-related diseases. A minimal amount of microorganisms is sufficient to form a biofilm outside the implants. Most infections are caused by microorganisms of the skin flora. During or immediately after neurosurgery, the implant becomes infected with microorganisms because of pauses in wound healing. In around 60% of instances, implant-related infections manifest early (within the first few weeks following surgery), whereas the remainder manifests later due to low-grade infections or direct spread from nearby infections to the implants due to soft tissue injury. Other than ventriculoatrial cerebrospinal fluid shunts, neurosurgical implants are often not involved via the hematogenous route (13).
Biofilm creation is an essential feature of prokaryotic endurance and has been detected in almost all subtypes of bacteria (excluding intracellular parasites such as Mycoplasma sp. and Chlamydia sp.), including bacteria linked to neurosurgical device-associated infections, such as S. aureus, Staphylococcus epidermidis, Pseudomonas aeruginosa, and Streptococcus sp. In contrast to the expression patterns of comparable subtypes in planktonic settings, the gene expression pattern in biofilm-forming bacteria is distinct. Recently, extreme efforts have resulted in the identification of new genes that are generated, especially in biofilm (14-19).
Recent research has revealed that bacterial biofilms play a major role in neurosurgical device-related infections. Since the idea of biofilms is unfamiliar to mainstream practitioners, it is essential to understand that conventional pharmacological treatments and host protection processes targeted at treating or conquering free-floating bacteria are useless versus immobile bacteria located in a biofilm. Bacterial biofilms are complicated and bound to the surface constructions comprised of extruded extracellular matrix. Microenvironments and several diverse bacterial phenotypes coexist inside this substance's microbial population. Each bacterial subtype in the biofilm demonstrates a unique algorithm for gene expression dependent on nutrient accessibility and waste transfer. This adaptability gives the whole biofilm a major existence advantage over each bacterial cell that comprises it. Consequently, it is feasible to consider the biofilm as a multicellular entity, comparable with metazoan eukaryotic organisms. Biofilms could be substantially more resilient than planktonic bacteria or bacteria that are freely floating and are the leading source of device-associated infections (20).
In this context, immunoinformatics could also be utilized to target biofilms, which are an important issue with neurosurgical implants. In silico has been widely applied to various conditions associated with bacterial infections (21, 22). It is intriguing that these immune-mediating candidates could be administered both as prophylactic options in at-risk populations as well as after being affected by the condition. Further research assessing the practicality of targeting major bacteria responsible for neurosurgical infection in high-risk individuals by the in silico approach is suggested.
3.3. Insights for Future Research; Role of COVID-19 Pandemic, Lifestyle Changes, and Interdisciplinary Immunoinformatics Research
In recent years, computational biology has facilitated research efforts by rapidly screening and designing drug candidates. Immunoinformatics helps to design immune-mediating candidates and evaluate the efficacy of candidates in silico. As discussed, neurosurgical practice desperately needs novel interdisciplinary approaches to develop and validate therapeutics.
Neurosurgery, one of the most scientifically sophisticated medical specialties, is rapidly adopting the most recent scientific advancements to treat CNS tumors. The survival rate for primary cerebral malignancies, for example; glioblastoma multiforme (GBM) or, less frequently, primary central nervous system lymphoma (PCNSL), is disheartening, although a great deal of effort has been dedicated to performing clinical studies. In recent years, cancer treatment and study have turned their attention to focused methods and personalized treatments. The creation of innovative and efficacious blood-brain barrier (BBB) medication delivery techniques to target malignant cells and TME mediators, such as the immune system is the primary theme that may be combined to improve the area (23).
During the current COVID-19 pandemic, Coronavirus patients worldwide topped 200 million, with an estimated 4.5 million deaths in 2021. Despite several attempts, there is no cure for this illness. The neurosurgical and neuro-oncological treatment of CNS cancer patients must be rapidly altered to meet the future demands of cancer patients influenced by COVID-19, according to these data. Neovascularization is a defining feature of CNS tumors. A patient's chance of developing infection via Severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) may be related to augmented ACE2 expression on endothelial cells and altered immunological feedback (24). In addition, cancer treatments and TME can result in vascular and immunosuppressive events, with ACE2 levels serving as a mediator in cancer cases. Malignant cells with changed immunogenic properties might cause immunological cells to yield immunosuppressive mediators, such as VEGF, TGF-, IL-10, PGE2, and iNOS, thereby impeding the cytotoxic reaction and proliferation of T-cells and resulting in the emergence of an inflammation-suppressive phenotype (such as Tregs, M2 macrophages). The Immunosuppressor microenvironment may cause the activation/polarization of anti-inflammatory M2 TAMs and naive DCs (25). However, cancer and COVID-19 cases might also have affected immunological and inflammatory interactions, including an increase in the production of IL-6 and IL-2 receptors and potential modifications in the pro-thrombotic state, for example, an increased prothrombin period. The initiator of the inflammatory response in COVID-19 needs to be further investigated (24). Immunoinformatics can help to tweak the current therapies and design new ones rapidly in the short time available during the current pandemic. Also, the in silico approach has been utilized to target the SARS-CoV-2 virus, resolving the problems caused by the pandemic within the neurosurgical field.
As mentioned, a multi-faceted approach to neurosurgical cases could lead to major advancements. Inflammation mediating therapies, due to their versatile implication in CNS conditions such as tumors and infections, and even viral infections such as COVID-19, maybe a central hub to target. Immunoinformatics can simulate the immune response shifting (e.g., by using the c-IMMSIM server), indicating another way it can benefit the neurosurgical field. Inflammasomes are pivotal complexes in the inflammatory response, which could be targeted in different neurological and non-neurological disorders (26, 27). Last, it is important that modifiable lifestyle changes are taken into account. Addiction has been linked to GBM in different contexts. A study suggested addiction and GBM development could be linked. The reported case corroborated the gliomagenesis influence of long-lasting various drug abuse, as well as its suppressive influence on tumor cell growth (28).
On the other hand, another experiment highlighted that methadone could not alleviate GBM progression. Methadone was proven to be only toxic to GBM cells at statistically significant doses but clinically irrelevant in vitro, where it was efficacious in causing necrosis and apoptosis. In contrast to TMZ, only doxorubicin was discovered to have sensitizing effects, which depend on the cellular lineage and the given drug dose. As a result, the results of this work did not advocate for the consumption of methadone in the therapy of GBM (29).
Overall, the good preliminary evidence on the role of in silico techniques and their tremendous speed advantage should warrant future studies to design immune-mediating candidates targeting CNS infections and tumors. The immunoinformatics approach should also be considered during a rapidly evolving pandemic such as COVID-19.
4. Conclusions
While the field of neurosurgery has advanced significantly, there is an emerging need for interdisciplinary research. This is because many CNS cancers remain hard to treat with traditional resection. Additionally, there are important adverse events associated with neurosurgical operations involving implants. Immunoinformatics attempts have been carried out to analyze the molecular basis and design vaccines against CNS tumors. However, these efforts are only in their preliminary phase. The speed advantage of the in silico approach is particularly valuable during the current COVID-19 pandemic, which affects CNS tumors' neuroimmunology and Neuro-Oncology practice. Finally, for future research, the mediation of immune response with the help of computational immunology to target CNS infections associated with neurosurgery may be a novel approach.