1. Background
Postoperative shivering (PS) is the most frequent complication during the recovery period after general anesthesia (1). Postoperative shivering reduces heat loss by involuntary skeletal muscle contractions and peripheral vasoconstriction. By preservation of heat, shivering causes more oxygen consumption, energy expenditure, production of carbon dioxide, increase in cardiac output, and elevation in intraocular pressure, can lead to lactic acidosis, tachycardia, and disturbs oxygen saturation monitoring, electrocardiogram (ECG), and blood pressure. It may cause difficulties in the recovery from general anesthesia, particularly in patients with cardiac and pulmonary problems. Shivering is an unpleasant experience, being able to bother more than surgical pain in some cases (2-5); however, the exact treatment and prophylactic medications need to be clarified (2). Shivering is a remarkable cause of distress after general anesthesia. Unfortunately, numerous studies aimed at developing the best way of prophylaxis for postanesthetic shivering have not identified a gold-standard pharmacologic agent (6-8).
Dexmedetomidine is a strong alpha-2 receptor agonist with sedative and also amnesis properties. It has been reported previously in several clinical studies that intrathecal or intravenous dexmedetomidine may prevent shivering (3, 9-11), but this effect remains controversial and has not been clearly explained. Although debatable, the prophylactic effect of dexmedetomidine on PS has been evaluated (3, 8, 12).
Prevention of PS has a tremendous effect on patient satisfaction and the reduction of complications caused by shivering. As mentioned previously, not only the efficacy of different agents previously used for preventing PS is unobvious, but the administration strategies and other potential complications are also unclear. In this study, we intend to evaluate the effect of dexmedetomidine on shivering appendectomy patients.
Postoperative shivering, as a heat loss reduction function, is considered the most frequent complication after general anesthesia. Shivering can lead to an inappropriate or even dangerous situation in patients and, therefore, should be considered.
2. Objectives
In this study, we intend to evaluate the preventive effect of dexmedetomidine on reducing shivering after appendectomy in patients undergoing general anesthesia.
3. Methods
The present research was a double-blind, randomized controlled trial approved by the Institutional Clinical Research Ethics Committee (Kurdistan University of Medical Sciences), and patients participated in this study with full knowledge and signed informed consent. Also, this study was registered at the Iranian Registry of Clinical Trials (IRCT20120801010471N4) and Ethics Committee (IR.MUK.REC.1395.385). Ninety patients underwent general anesthesia for appendectomy with inclusion criteria, including the American Society of Anesthesiologists (ASA) classifications 1 and 2 and an age range of 15-65 years. Exclusion criteria included peritonitis, malignant hyperthermia, epilepsy, anaphylaxis, cardiac disease, vascular disease, dyspnea, diabetes mellitus, renal failure, liver disease, psychiatric disorders, addiction, and alcohol consumption.
Before the start of the study, the study participation conditions were explained to the patients. Their questions were answered, and there was a possibility of withdrawing from the study if they did not intend to participate in the study. They were ensured that their information would be kept confidential and analyzed with codes and anonymously. Those willing to participate in the study were asked to read and sign the written informed consent. Medicines were prepared by the anesthesiologist in invisible syringes, and the blind anesthesiologist injected the drugs.
In this study, the response rate of PS was a general anesthetic method as a qualitative variable with five levels (zero to four). Ninety selective patients participated in this study. Our sample size was calculated to be 45 patients in each group, considering a significance level of 95%, a test power of 90%, and an effect size of 0.85. We started our study in 2017-04-21 and ended it in 2018-05-03.
Randomization of patients was performed using the www.random.org website from the numbers of sequence generator option; numbers 1 to 15 were randomly placed in groups A and B. In this way, the grouping of the first 15 eligible patients was determined; for example, in randomization, number 14 in column B means that the 14th disease eligible to enter the study will be placed in group B, etc. Randomization was carried out again for the next 15 patients. This process continued until we reached the desired sample size.
The included patients were randomly assigned to the dexmedetomidine and placebo groups. Random allocation and provision of anesthetic medicines were performed by an anesthetist who did not interfere with the procedure and outcome measurements.
We studied patients referring to Besat Hospital in the city of Sanandaj for surgery with one and two physical statuses regarding the division of the ASA using a randomized method. The questionnaire was completed by a medical student under a research associate's anesthesiologist, both blind.
The anesthetic induction procedure was quite similar in both groups. Before induction of anesthesia, fluid therapy was performed with a ringer at a rate of 7cc/kg within 5-7 minutes. Pre-oxygenation was performed with 5 liters of oxygen using anesthetic masks for all patients. First, fentanyl 2μ/kg was prescribed as premedication, and 5 mg/kg of sodium thiopental and 1.5 mg/kg of succinylcholine were later prescribed as the anesthetic agent. Maintenance of anesthesia in both groups included the minimum alveolar concentration (MAC) of isoflurane and oxygen+N2O mixture of equal amounts (3 Lit/min). All patients underwent synchronized intermittent mode of ventilation (SIMV) with a tidal volume of about 6 mL/kg, a frequency rate of 12 to 14 per minute, and a positive end-expiratory pressure (PEEP) of 3. The intraoperative fluid (4 cc/ kg/ h) was injected as maintenance fluid plus maintenance fluid + 3cc/kg per cc of blood loss. Hemorrhage was measured based on the blood mass of sterile gases together with the amount of blood loss in the suction. The temperature of all ringer fluids was the same and supplied by the common room for maintenance of the operating room sera. The room temperature was 25 - 28° C, and a serum was selected to measure the temperature on a daily basis randomly. Fentanyl 1μ/kg was injected as the opioid, based on the BIS criteria. Atracurium 0.2 mg/kg was injected as the intraoperative relaxant. The control group received 10 cc of distilled sterilized intravenously within 10 - 20 minutes before anesthetic induction.
In the intervention group, 0.5 μ/kg of dexmedetomidine was injected intravenously 20 minutes before extubation for 10 minutes (1μ/kg diluted in 10 mL of sterile distilled water). The control group received 10 cc of distilled sterilized intravenously within 10 - 20 minutes before extubation. Patients' body temperature and noninvasive blood pressure (NIBP) are measured at the baseline. Also, common monitoring included ECG (Lead II), pulse oximetry, NIBP, and capnography for all patients.
The severity of shivering was evaluated based on the visual analog scale (VAS) criteria as the primary outcome.
The scale used was as follows:
0 = No shivering;
1 = Observation of one or more of the following: Piloerection, peripheral vasoconstriction, peripheral cyanosis without other causes, and no visible muscular activity;
2 = Visible muscular activity confined to 1 muscle group;
3 = Visible muscular activity in more than 1 muscle group;
4 = Gross muscular activity involving the whole body.
Shivering was evaluated immediately and then at 5, 15, 30, and 60 minutes after general anesthesia. The shivering rate was evaluated based on the following criteria. Meperidine (25 mg) was injected intravenously in case of a shivering score greater than 2. The room temperature was maintained at 22°C during the anesthesia, no heating system was used for patients, and all surgical procedures were performed in the general surgery room.
In this study, the hypotension was defined as MAP ≤ 60mmHg or more than 20% reduction in the baseline rate. In the event of hypotension, 5 mg of ephedrine was prescribed intravenously. Atropine was injected intravenously (0.2 mg/kg) to the patient in case of bradycardia (heart rate less than 50) or more than 20% reduction in heart rate compared to the baseline rate.
Data were entered into SPSS V.23 after data collection and checked for missing data. Descriptive statistics, including mean, standard deviation, and frequency, were used to describe the results. The variables were compared between the two groups using the chi-square and Fisher’s exact tests for categorical variables and the Student's t-test and Mann–Whitney U test for parametric and nonparametric variables, respectively. Also, repeated measures analysis of variance (ANOVA) was used to evaluate parameters over time. The significance level was considered < 0.05 in all tests.
4. Results
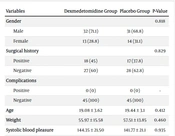
A total of 90 patients were enrolled in our study, with 63 (70%) males and 27 (30%) females. The gender difference was not significant in the two groups (P = 0.818). The mean ± SD on age in all participants was 19.38 ± 3.24 (age range = 18 to 56). The mean age was not significant in patients and the control group (P = 0.412).
There were no significant differences between the two groups in terms of the weight, temperature of serum for fluid therapy, fluid volume, perioperative body temperature, preoperative body temperature, and room temperature (Table 1).
| Variables | Dexmedetomidine Group | Placebo Group | P-Value |
|---|---|---|---|
| Gender | 0.818 | ||
| Male | 32 (71.1) | 31 (68.8) | |
| Female | 13 (28.8) | 14 (31.1) | |
| Surgical history | 0.829 | ||
| Positive | 18 (45) | 17 (37.8) | |
| Negative | 27 (60) | 28 (62.8) | |
| Complications | |||
| Positive | 0 (0) | 0 (0) | - |
| Negative | 45 (100) | 45 (100) | |
| Age | 19.08 ± 3.62 | 19.44 ± 3.1 | 0.412 |
| Weight | 55.97 ± 15.58 | 57.51 ± 13.85 | 0.460 |
| Systolic blood pleasure | 144.35 ± 21.50 | 141.77 ± 21.1 | 0.935 |
| Diastolic blood pleasure | 86.46 ± 18.41 | 84.53 ± 14.86 | 0.589 |
| Body's temperature | 37.41 ± 0.89 | 37.22 ± 0.67 | 0.975 |
| Surgery time (min) | 75.23 ± 12.65 | 69.74 ± 10.64 | 0.0473 |
| Preoperative fluid volume | 287.14 ± 69.03 | 279.01 ± 78.96 | 0.623 |
| Perioperative fluid volume | 542.9 ± 136.99 | 532.93 ± 139.34 | 0.736 |
| Temperature of serum for fluid therapy | 28.8 ± 32.1 | 24 ± 0 | 0.315 |
| Preoperative body temperature | 37.64 ± 1.53 | 37.76 ± 0.45 | 0.540 |
| Perioperative body temperature | 36.6 ± 0.42 | 36.54 ± 0.43 | 0.418 |
| Room temperature | 25 ± 2 | 24.9 ± 0.14 | 0.310 |
a Frequency and analysis of variance tests were used.
b Values are presented as No. (%) or mean ± SD.
The severity of shivering was significantly lower in the dexmedetomidine group (60%) than in the placebo group (P < 0.001). Also, other criteria for shivering in both groups of patients are shown in Table 2.
| Severity of Shivering | Placebo Group | Dexmedetomidine Group | P-Value |
|---|---|---|---|
| No shivering (0) | 0 (0) | (60) 27 | < 0.001 |
| Idiopathic partial cyanosis of extremities/hair stiffening (1) | 6 (13.3) | 13 (28.9) | |
| Muscular shaking in a single group of muscles (2) | 5 (11.1) | 1 (2.2) | |
| Muscular shaking in more than one group of muscles (3) | 15 (33.3) | 2 (4.4) | |
| Muscular shaking visible in the whole body (4) | 19 (42.5) | 2 (4.4) | |
| Total | 45 | 45 |
a Values are presented as No. (%).
Table 3 shows no significant differences according to gender/surgical history and the severity of shivering between the two groups (P > 0.05). Also, no significant relationship was found between age, perioperative fluid volume, preoperative body temperature/perioperative body temperature, and the severity of shivering in any of the study groups (Table 4).
| Groups and Variables | Severity of Shivering | P-Value | ||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | ||
| Placebo Group | ||||||
| Gender | 0.815 | |||||
| Male | 0 (0) | 5 (11.1) | 3 (6.6) | 10 (22.2) | 14 (31.1) | |
| Female | 0 (0) | 1 (2.2) | 2 (4.4) | 5 (11.1) | 5 (11.1) | |
| Dexmedetomidine Group | ||||||
| Gender | 0.220 | |||||
| Male | 20 (44.4) | 7 (15.5) | 0(0) | 2 (2.2) | 2 (2.2) | |
| Female | 7 (15.5) | 6 (13.3) | 1 (2.2) | 0(0) | 0(0) | |
| Negative | 17 (37.7) | 9 (20) | 0(0) | 1 (2.2) | 1 (2.2) | |
a Values are presented as No. (%).
| Groups | Variables | Severity of Shivering | P-Value | ||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | |||
| Placebo group | Age | 18.3 ± 3.5 | 18.8 ± 2.6 | 20.2 ± 4.3 | 18.3 ± 3.3 | 19 ± 3.6 | 0.542 |
| Dexmedetomidine group | 19.5 ± 3.4 | 19 ± 3.4 | 23 ± 11.1 | 20 ± 4.2 | 19 ± 1.1 | 0.807 | |
| Placebo group | Perioperative fluid volume | 0 ± 0 | 547 ± 170 | 474 ± 125 | 595 ± 152 | 494 ± 109 | 0.138 |
| Dexmedetomidine group | 535 ± 155 | 551 ± 112 | 586 ± 59 | 584 ± 50 | 527 ± 121 | 0.981 | |
| Placebo group | Preoperative body temperature | 0 ± 0 | 37.5 ± 0.5 | 38 ± 0.3 | 37.2 ± 0.42 | 38 ± 0.37 | 0.591 |
| Dexmedetomidine group | 37.7 ± 0.44 | 37.7 ± 0.52 | 38 ± 0.4 | 37.5 ± 0.7 | 38 ± 0 | 0.834 | |
| Placebo group | Perioperative body temperature | 0 ± 0 | 36.4 ± 0.037 | 36.4 ± 0.41 | 36.5 ± 0.45 | 36.6 ± 0.45 | 0.704 |
| Dexmedetomidine group | 36.6 ± 0.41 | 36 ± 0 | 36.5 ± 0.7 | 36.5 ± 0.5 | 36.7 ± 0.42 | 0.625 | |
a Values are presented as mean ± SD.
5. Discussion
Different medications reduce body temperature. General anesthesia or deep sedation can lead to the complete elimination of shivering. Previous studies have shown that using α2 agonists such as dexmedetomidine can reduce shivering and be a simple drug intervention; however, it is not suitable for patients with acute ischemia or stroke (13). The results of our study were similar to the results of these articles, revealing a statistically significant difference between the dexmedetomidine and control groups (P = 0.0001).
Shivering is a state of changing continuously and without intended contraction of the skeletal muscle, with potentially increasing discomfort in patients (14).
A meta-analysis refers to using dexmedetomidine as an anti-shivering agent, aiming to determine the factors that might influence its effectiveness. Based on the data of the relevant trials, we found that 0.5 µg/kg of dexmedetomidine sufficiently and effectively prevented PS (3). These data support the results of our study.
The present study examined the effects of one of the α2-agonist variants (dexmedetomidine) in reducing shivering after general anesthesia in appendectomy surgery. The present study showed a significant relationship between the use of dexmedetomidine and a reduction in shivering rate. This finding was consistent with the new meta-analysis’s data on the use of dexmedetomidine (0.5 µg/kg) (3, 6). In another study, tramadol was used as an anti-shivering drug, but its complications were greater than those of dexmedetomidine (15).
Our results suggest that 0.5 µg/kg of dexmedetomidine provides effective prophylaxis against PS and has an analgesic effect. Though the potential for intraoperative requirement for atropine, sedation in the immediate recovery period, and delayed extubating time with dexmedetomidine were noted, there were no major clinical impacts on the overall recovery from anesthesia (16).
The administration of prophylactic intravenous dexmedetomidine reduces the incidence of shivering in patients undergoing general anesthesia (8).
Although the results of our study revealed no significant difference between the control and intervention groups regarding the severity of shivering according to gender (P > 0.05), other studies did not assess the relevance between these two items.
Our study supported that dexmedetomidine could reduce shivering in general anesthesia, but it does not affect the severity of shivering. This result is in concordance with other studies (3, 8, 11, 15).
In our study, no significant relationship was found between gender, education level, and marital status (P-value < 0.05). An interesting point in our study was that although the use of dexmedetomidine reduced the amount of shivering in general anesthesia, it was not effective on the severity of shivering, and there was no difference between the intervention and control groups in this regard. One of the benefits of our study was the use of dexmedetomidine in emergency surgeries. Although regional methods such as spinal and epidural are used in appendectomy surgery in many cases, in our age group, the use of these methods is sometimes associated with restrictions such as dissatisfaction among patients or their parents. Therefore, this study could be the basis for further studies in emergency cases.
5.1. Conclusions
In conclusion, the use of dexmedetomidine reduced shivering after appendectomy; however, dexmedetomidine did not affect the severity of PS. More studies are required in this area, particularly on regional anesthetic techniques.